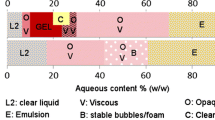
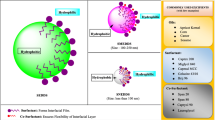
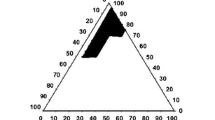
Abstract
Purpose
The goal of this research was to study the molecular interactions in situ between actives and surfactants in self-emulsifying delivery systems (SEDDS) in order to illuminate the factor(s) and how they influence the drug performance in these systems.
Method
We have developed an approach to evaluate the mechanism of performance of SEDDS formulations using an in situ Raman technique. Self-emulsifying delivery systems for seven actives with different physicochemical properties were formulated. One gram of the SEDDS was dispersed in 100 mL of media, and the precipitation behavior of the actives was noted. Using an immersion probe, these dispersions were tested continuously and spectra were obtained to determine the drug-excipient interactions.
Results
The changes in the molecular vibrational peaks confirmed that the drug-TPGS hydrogen bonding was responsible for maintaining the drug in solution. The interaction between Labrasol and the actives was weak and broke upon dispersion leading to precipitation of the drug. Also, the hydrogen bond donor strength of the functional group gave a good indication of the bond strength between TPGS and the drugs which determined the performance of the actives in the system.
Conclusion
It could be suggested that the hydrogen bond strength correlates well to the precipitation behavior from the SEDDS dispersions. Thus, studying the structures and physicochemical properties of the drug candidates and the excipients could significantly minimize the steps involved in developing successful lipid-based delivery systems.








Similar content being viewed by others
References
Hauss DJ. Oral lipid-based formulations. Enhancing the bioavailability of poorly water-soluble drugs. Informa Healthcare, Inc. New York, 2007, Vol 170.
Reddy S, Katyayani T, Navatha A, Ramya G. Review on self-micro emulsifying drug delivery systems. Int J Res Pharm Sci. 2011:382–92.
Bevernage J, Brouwers J, Brewster ME, Augustijns P. Evaluation of gastrointestinal drug supersaturation and precipitation: strategies and issues. Int J Pharm. 2013;453(1):25–35. https://doi.org/10.1016/j.ijpharm.2012.11.026.
Brouwers J, Brewster ME, Augustijns P. Supersaturating drug delivery systems: the answer to solubility-limited oral bioavailability? J Pharm Sci. 2009;98(8):2549–72. https://doi.org/10.1002/jps.21650.
Thomas N, Mullertz A, Graf A, Rades T. Influence of lipid composition and drug load on the in vitro performance of self-nanoemulsifying drug delivery systems. J Pharm Sci. 2012;101(5):1721–31. https://doi.org/10.1002/jps.23054.
Thomas N, Holm R, Mullertz A, Rades T. In vitro and in vivo performance of novel supersaturated self-nanoemulsifying drug delivery systems (super-SNEDDS). J Control Release. 2012;160(1):25–32. https://doi.org/10.1016/j.jconrel.2012.02.027.
Anby MU, Williams HD, McIntosh M, Benameur H, Edwards GA, Pouton CW, et al. Lipid digestion as a trigger for supersaturation: in vitro and in vivo evaluation of the utility of polymeric precipitation inhibitors in self emulsifying drug delivery systems. Mol Pharm. 2012;9(7):2063–79. https://doi.org/10.1021/mp300164u.
Devraj R, Williams HD, Warren DB, Porter CJH, Powton CW. Choice of nonionic surfactant used to formulate type IIIA self-emulsifying drug delivery systems and the physicochemical properties of the drug have a pronounced influence on the degree of drug supersaturation that develops during in vitro digestion. J Pharm Sci. 2014;103(4):1050–63. https://doi.org/10.1002/jps.23856.
Williams HD, Trevaskis NL, Charman SN, Shanker RM, Charman WN, Pouton CW, et al. Strategies to address low drug solubility in discovery and development. Pharmacol Rev. 2013;65(1):315–499. https://doi.org/10.1124/pr.112.005660.
Cuine JF, McEvoy CL, Charman WN, Pouton CW, Edwards GA, Benameur H, et al. Evaluation of the impact of surfactant digestion on the bioavailability of danazol after oral administration of lipidic self-emulsifying formulations to dogs. J Pharm Sci. 2008;97(2):995–1012. https://doi.org/10.1002/jps.21246.
Lee DH, Yeom DW, Song YS, Kang MJ, Choi Y-W. Improved oral absorption of dutasteride via Soluplus®-based supersaturable self-emulsifying drug delivery system (S-SEDDS). Int J Pharm. 2015;478(1):341–7. https://doi.org/10.1016/j.ijpharm.2014.11.060.
Piao Z-Z, Choe J-S, Oh KT, Rhee YS, Lee BJ. Formulation and in vivo human bioavailability of dissolving tablets containing self-nanoemulsifying itraconazole solid dispersion without precipitation in simulated gastrointestinal fluid. Eur J Pharm Sci. 2014;51:67–74. https://doi.org/10.1016/j.ejps.2013.08.037.
Song WH, Yeom DW, Lee DH, Song SH, Choi YW. In situ intestinal permeability and in vivo oral bioavailability of celecoxib in supersaturating self-emulsifying drug delivery system. Archives Pharm Res. 2014;37(5):626–35. https://doi.org/10.1007/s12272-013-0202-7.
Warren D, Christel AS, Bergström BH, Porter CJH, Pouton CW. Evaluation of the structural determinants of polymeric precipitation inhibitors using solvent shift methods and principle component analysis. Mol Pharm. 2013;10(8):2823–48. https://doi.org/10.1021/mp300576u.
DiNunzio JC, Miller DA, Yang W, McGinity JW, Williams RO. Amorphous compositions using concentration enhancing polymers for improved bioavailability of itraconazole. Mol Pharm. 2008;5(6):968–80. https://doi.org/10.1021/mp800042d.
Ziller KH, Rupprecht H. Control of crystal growth in drug suspensions: 1 design of a control unit and application to acetaminophen suspensions. Drug Dev Ind Pharm. 1988;14(15-17):2341–70. https://doi.org/10.3109/03639048809152019.
Chen C, Xie X, Li Y, Yan Z, Yang X. Influence of different polymers on crystallization tendency and dissolution behavior of cilnidipine in solid dispersions. Drug Dev Ind Pharm. 2014;40(4):441–51. https://doi.org/10.3109/03639045.2013.767825.
Paudel A, Neis E, Mooter GVD. Relating hydrogen-bonding interactions with the phase behavior of naproxen/PVP K 25 solid dispersions: evaluation of solution-cast and quench-cooled films. Mol Pharm. 2012;9:3307–17.
Raut S, Karzoun B, Atef E. Using in situ Raman spectroscopy to study the drug precipitation inhibition and supersaturation mechanism of vitamin E TPGS from self-emulsifying drug delivery systems (SEDDS). J Pharm Biomed Anal. 2015;109:121–7. https://doi.org/10.1016/j.jpba.2015.02.027.
Hassan MA, Sheikh Salem M, Sallam E, Al-Hindawi MK. Preparation and characterization of a new polymorphic form and a solvate of glibenclamide. Acta Pharm Hung. 1997;67(2-3):81–8.
Fini A, Ospitali F, Zoppetti G, Puppini N. ATR/Raman and fractal characterization of HPBCD/progesterone complex solid particles. Pharm Res. 2008;25(9):2030–40. https://doi.org/10.1007/s11095-008-9593-4.
Kasal A, Budesinsky M, Griffiths W. Spectroscopic methods of steroid analysis. Makin HLJ and Gower DB Steroid Analysis Springer Science. 2010;
Wojciech C, Baranska M. Carbamazepine polymers: theoretical and experimental vibrational spectroscopy studies. Vib Spectrosc. 2013;65:12–23.
Rodrigues NVS, Cardoso EM, Andrade MVO, Donnici CL, Sena MM. Analysis of seized cocaine samples by using chemometric methods and FTIR spectroscopy. J Braz Chem Soc 2014; 24:507–517.
Neville GA, Beckstead HD, Shurvell HF. A fourier transform-Raman and infrared vibrational study of delorazepam, fludiazepam, flurazepam and tetrazepam. J Pharm Sci. 1994;83(2):143–51. https://doi.org/10.1002/jps.2600830207.
Gunasekaran S, Thilak R, Ponnusamy S. Vibrational spectra and normal coordinate analysis of diazepam, phenytoin and phenobarbitone. Spectrochim Acta. 2006;65(5):1041–52. https://doi.org/10.1016/j.saa.2006.01.037.
Cruz-Cabeza AJ, Schwalbe CH. Observed and predicted bond motifs in crystal structures of hydantoins, dihydrouracils and urasils. New J Chem. 2012;36(6):1347–54. https://doi.org/10.1039/c2nj21060f.
Todeschini R and Consonni V. Methods and principles in medicinal chemistry in Mannhold R, Kubinyi H, Timmerman H. 3rd ed. Handbook of molecular descriptors Wiley-VCH 2008; 11:223–224.
Gao P, Akrami A, Alvarez F, Hu J, Li L, Ma C, et al. Characterization and optimization of AMG 517 supersaturatable self-emulsifying drug delivery system (S-SEDDS) for improved oral absorption. J Pharm Sci. 2009;98(2):516–28. https://doi.org/10.1002/jps.21451.
Chen ZQ, Liu Y, Zhao JH, Wang L, Feng NP. Improved oral bioavailability of poorly water-soluble indirubin by a supersaturatable self-microemulsifying drug delivery system. Int J Nanomedicine. 2012;7:1115–25. https://doi.org/10.2147/IJN.S28761.
Acknowledgements
The authors would like to thank Gattefosse for providing gift samples of Lauroglycol®, Labrasol®, and Transcutol® HP.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest
Additional information
Chemical Compounds Studied in This Article:
Indomethacin (PubChem CID 3715); Glyburide (PubChem CID 3488)Probucol (PubChem CID 4912)Progesterone (PubChem CID 5994)Carbamazepine (PubChem CID 2554); Lidocaine (PubChem CID 3676)Lorazepam (PubChem CID 3958)Phenytoin (PubChem CID 1775)
Rights and permissions
About this article
Cite this article
Raut, S., Atef, E. Characterization of Self-Emulsifying Drug Delivery Systems Using In Situ Raman Spectroscopy to Study the Precipitation Inhibition Mechanism of Poorly Water-Soluble Drugs. J Pharm Innov 13, 144–154 (2018). https://doi.org/10.1007/s12247-018-9315-3
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12247-018-9315-3