Abstract
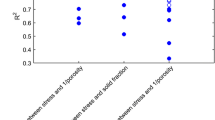
In pharmaceutical development, powder flow characteristics are notoriously difficult to predict. Although tests exist that aim to determine the flowability of powders, many have not been definitively correlated with a powder’s performance in downstream processes. Part of the challenge is that powder flow must occur over a broad range of consolidating stresses, including low-stress flow regimes such as the filling of a die cavity in a tablet press and high-stress flow regimes such as the deformation associated with tablet compression. The objective of this work was to characterize several placebo formulations utilizing a variety of standardized and non-standardized experiments and contrasting those results with downstream tablet compression performance. Angle of repose, bulk and tap density, Hausner ratio, Carr’s compressibility index, a visual powder flow assessment, a mass flow rate test, Flodex, and powder shear testing were evaluated on several different blends of placebo powders to determine a predictive test for downstream process performance. The placebo powders consisted of a standard base unit formula, mixed with various levels of four different “pseudo-active pharmaceutical ingredients (APIs)” (i.e., excipients that mimic poorly flowing active pharmaceutical ingredients). All formulations were evaluated by the individual flow tests and then compressed on a rotary tablet press. Formulations were deemed failures if the relative standard deviation of tablet weight was >2 %, material stuck to punches (low lubrication), or exhibited ejection forces high enough to trigger automatic shutdown of tablet press. The 2 % RSD limit is based on internally developed best practices for transferring tablet compression processes from development into manufacturing. The results of the tablet compression process were then compared to the results of the flowability tests to detect any correlations. The Hausner ratio and powder shear test results discriminated marginally between successful and failed batches and were highly correlated with each other.









Similar content being viewed by others
Notes
An exception occurred for lot (50 % dicalcium phosphate), where the press was unintentionally run at 100 rpm.
References
Geldart D, Abdullah Ec, Verlinden A. Characterisation of dry powders. Powder Technol. 2008;190:70–4 .Web. 16 June 2015
Traina K, Cloots R, Bontempi S, Lumay G, Vandewalle N, Boschini F. Flow abilities of powders and granular materials evidenced from dynamical tap density measurement. Powder Technol. 2003;235:842–52.
Emery E, Oliver J, Pugsley T, Sharma J, Zhou J. Flowability of moist pharmaceutical powders. Powder Technol. 2009;189:409–15.
Thalberg K, Lindholm D, Axelsson A. Comparison of different flowability tests for powders for inhalation. Powder Technol. 2004;143:206–13.
Leturia M, Benali M, Lagarde S, Ronga I, Saleh K. Characterization of flow properties of cohesive powders: a comparative study of traditional and new testing methods. Powder Technol. 2014;253:406–23.
Shah RB, Tawakkul MA, Khan MA. Comparative evaluation of flow for pharmaceutical powders and granules. AAPS Pharm SciTech. 2008;9(1):250–8. doi:10.1208/s12249-008-9046-8.
Søgaard SV, Pedersen T, Allesø M, Garnaes J, Rantanen J. Evaluation of ring shear testing as a characterization method for powder flow in small-scale powder processing equipment. Int J Pharm. 475:315–23.
Zhao J, Burt HM, Miller RA. The Gurnham equation in characterizing the compressibility of pharmaceutical materials. Int J Pharm. 2006;317:109–13.
Kushner J, Moore F. Scale-up model describing the impact of lubrication on tablet tensile strength. Int J Pharm. 2010;399(1–2):19–30.
Cunningham Jc, Sinka Ic, Zavaliangos A. Analysis of tablet compaction. I. Characterization of mechanical behavior of powder and powder/tooling friction. Journal of Pharmaceutical Sciences J Pharm Sci. 2004;93(8):2022–39 .Wiley Inter Science. Web. 9 July 2015. <www.interscience.wiley.com>
Aburub A, Buckner IS, Mishra D. Use of compaction energetics for understanding particle deformation mechanism. Pharmaceut Develop Technol. 2007;12:405–14.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Zettler, A., Hilden, J., Koenig, M. et al. Evaluation of Small-Scale Powder Flow Characterization Tests in the Prediction of Large-Scale Process Failures. J Pharm Innov 11, 189–199 (2016). https://doi.org/10.1007/s12247-016-9258-5
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12247-016-9258-5