Abstract
Salt marshes have ecological and economic value, but shoreline development, the increasing rate of sea-level rise, and other human impacts have caused significant loss of salt marshes. As a result, restoration of these ecosystems is widespread. For restoration and management to be effective, it is imperative to improve our understanding of marsh-building plants that serve as the ecological foundation of these habitats. Given the observed differences in characteristics between populations of smooth cordgrass, Spartina alterniflora, restoration plantings may impact the biodiversity and resilience of restored ecosystems. Understanding differences in the structural and functional outcomes of active planting of restoration sites will enable the long-term success of restoration efforts to be improved. Natural and restored salt marshes in Long Island Sound were studied in 2021–2022 for S. alterniflora genetics, biomass, stem morphology, and faunal community composition. The average genotypic diversity of S. alterniflora was more than 4 times higher in restored than in natural marshes, and differentiation between each restored site and natural sites decreased with time. No difference was observed in live S. alterniflora belowground biomass; however, mean dead belowground biomass in natural marshes was more than 3 times greater than in restored marshes. Marsh platform invertebrates differed between the restored and natural sites, with natural marsh edge habitats having 9 times higher density of Geukensia demissa and 3 times as many crab burrows than in restored marshes, but there was no detected difference in species richness or abundance of nekton at high tide. With restoration practitioners seeking resilient, self-sustaining ecosystems, it is important to evaluate whether restored marsh characteristics are consistent with those goals and modify restoration planning accordingly to incorporate genetics, structure, and function.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
As one of the most productive ecosystems on earth, salt marshes provide valuable ecosystem services on which we depend (Barbier et al. 2011). Despite this value, many salt marshes have been lost due to development, hydrological modifications, and other anthropogenic forces (Bromberg and Bertness 2005; Campbell et al. 2022). Beyond past losses, sea-level rise presents an increasing threat to salt marshes because they are found only in a narrow elevation range relative to the coast. If salt marshes are not able to gain elevation to match the increasing rate of sea-level rise, they will drown and convert to open water or mudflats. While the magnitude of loss to be expected is complex to predict and debated (Schuerch et al. 2018; FitzGerald and Hughes 2019; Fagherazzi et al. 2020; Coleman et al. 2022), as much as 60–91% of current salt marsh could be lost with predicted future rates of sea-level rise (Crosby et al. 2016). Given the cumulative magnitude of these threats, it is critical that we maximize the effectiveness and efficiency of the conservation, restoration, and management of these important ecosystems.
Salt marshes are one of the coastal ecosystems most commonly targeted for restoration (Grabowski et al. 2012). Salt marsh restoration projects rely on the establishment of the foundational plants that serve as ecosystem engineers for the marsh through planting or natural colonization. Some of the factors that contribute to their successful establishment remain poorly understood, leading to variable success among restoration efforts (Bayraktarov et al. 2016; Liu et al. 2024). One previously overlooked factor is the genetic structure of the plants themselves (Travis and Grace 2010; Hughes and Lotterhos 2014). Genetically diverse populations of ecosystem engineers have increased productivity and enhanced response to stressors across many ecosystems (Bischoff et al. 2010; Kettenring et al. 2014). Restored populations often, but not always, have lower genetic diversity than natural populations (Wei et al. 2023), but the relationship between diversity and ecosystem structure and function in restored populations remains unclear (McKay et al. 2005; Jones 2013). Genetic diversity within species is especially important in ecosystems where one or few plant species dominate the landscape in actual or near monoculture, such as the different elevation zones of many salt marshes (Hughes et al. 2014; Hughes 2014). In these instances, intraspecific diversity plays an analogous role to species diversity in the maintenance of ecological structure and function in the face of disturbance (Hughes et al. 2008). Work with the dominant marsh grass Spartina alterniflora in Louisiana (USA) found that the genetic diversity of naturally colonized restored populations was similar to that of natural marsh populations, enabling the restored sites to withstand disturbances similarly (Travis et al. 2002). However, these passive restoration projects may differ from those where direct planting of plugs of salt marsh plants occurs. These restoration projects may thus result in a genetic structure of the restored marsh S. alterniflora population that differs from nearby natural marshes (Kettenring et al. 2014) since individual plugs planted in close proximity may or may not be closely related genetically, whereas clonal expansion within a natural marsh by perennial species would tend to increase the genetic similarity of stems within a given area. As such, there is an opportunity to better incorporate our understanding of foundation species genetics into the implementation of salt marsh restoration to ensure their long-term persistence and function (Zerebecki et al. 2017; Noto and Hughes 2020; Zogg and Travis 2022).
Given the observed differences in growth form and other characteristics within and between different populations of S. alterniflora (Zerebecki et al. 2021), especially differences that occur latitudinally along the Atlantic coast of the USA (Crosby et al. 2017), the source of plants used for restoration may impact the resilience of the restored ecosystem as well as biodiversity of the marsh as a whole. Plant genetics and associated phenotypic variation have been shown to influence the associated animal community in other systems such as shrubs (Barbour et al. 2015), trees (Wimp et al. 2004; Evans et al. 2012), and herbaceous flowering plants (Johnson et al. 2009). These plant-animal linkages are likely especially strong for species reliant on S. alterniflora’s physical structure like gastropods, bivalves, and crustaceans (Zerebecki and Hughes 2023). Understanding differences in genetic diversity of S. alterniflora, but also the ecological impacts on the plant and animal community and the structure, function, and resilience of the ecosystem that results, will enable the long-term success of restoration efforts to be improved.
In the USA, many salt marsh restoration projects are led by state or local agencies (or their designees) charged with mitigating adverse impacts of other projects (e.g., transportation infrastructure). In many cases, marshes are restored by planting salt marsh plant plugs purchased from commercial nurseries. The impact of purchasing plants from sometimes distant or unknown geographic sources that may differ in their propagation methods on the characteristics of the restored marsh and the success of the restoration is often unknown, and in some cases is not considered in the restoration design or evaluation.
Due to the differences in implementation methods (e.g., planting versus passive colonization of plants), seascape context, differing restoration goals, and other factors, a variety of differences between natural and restored salt marshes have been observed. For some sites, these differences have been structural, such as in plant species composition (Mossman et al. 2012) or site topography (Lawrence et al. 2018). A meta-analysis of salt marshes restored through active planting also found multiple functional differences: restored marshes had lower soil carbon storage, lower primary productivity, and overall lower biodiversity than natural marshes, among other differences (Liu et al. 2024). Other studies have shown differences in nutrient cycling (Thompson et al. 1995) and secondary production dynamics (Rezek et al. 2017). The magnitude of these differences can sometimes be related to the age of restored sites; long timescales can be required—from several years to multiple decades—to reach structural or functional equivalency (Warren et al. 2002; Liu et al. 2024). These structural or functional differences can also translate into differences in the resiliency of these sites to sea-level rise. With the increasing rates of sea-level rise predicted, if restored sites take long time frames to achieve intended structure and function, then they may not reach resilience before they are submerged.
Here, we sought to explore the interactions between a foundation species’ morphology, genetics, and ecological function, through a study of 12 natural and restored salt marshes in the western Atlantic in Long Island Sound, along the coast of CT, USA. Through this study, we explored the growth and genetics of a foundation plant species and ecological function of natural and restored salt marshes to answer two key questions: (1) How will natural and restored marshes differ in their future resilience to sea-level rise, and (2) how can we learn from those differences to improve restoration practices? To answer these questions, measurements of S. alterniflora (genetics and growth parameters) and surveys of fauna were conducted; however, to tease apart the potential abiotic contributions to any differences observed that may be unrelated to genetics or the marsh’s status as natural or restored, a suite of abiotic variables were also assessed and a common garden study of S. alterniflora was conducted. Restored marshes were hypothesized to have lower S. alterniflora genetic diversity, lower plant biomass, and less biodiversity and faunal abundance than the natural marshes due to their relatively young age and expectations about restoration success from previous studies.
Methods
Study Area and Sampling Timing
The 12 study sites were located along the northern coast of Long Island Sound, USA, between Stratford and Groton, CT, USA, and included 6 natural and 6 restored salt marshes (Table 1). Restored marshes were selected with the help of restoration practitioners at the Connecticut Department of Transportation (CT DOT) and Sacred Heart University. Restored marshes were selected based on having a known source of plugs for S. alterniflora used during restoration, as well as their accessibility. The previous restoration activities at each of these 6 sites differed, such as some including hydrological modification, re-grading elevation, and Phragmites australis removal, while another included planting S. alterniflora to establish a new marsh in what had previously been a cobble beach after establishment of an artificial reef to attenuate wave-driven erosion. However, all 6 marshes shared the fact that they were planted with plugs of S. alterniflora sourced from offsite commercial vendors versus allowing natural colonization or transplanting plugs directly from a nearby marsh as is sometimes done in other restoration projects. Based on communication with the restoration practitioners for each site, the study area sampled at each is believed to be dominated by cover of S. alterniflora that resulted from the planted plugs. Natural marshes were selected based on their proximity to the restored marshes, presence of the study species (S. alterniflora), and availability of habitat for study. Potential study sites were assessed using aerial imagery, after which site visits were conducted to confirm the presence of the study species and accessibility.
Field sampling was conducted six times during the study period over approximately 2 weeks each in the spring, summer, and fall of the growing seasons of 2021 and 2022 (April 29–May 6, 2021, July 13–27, 2021, September 27–October 4, 2021, April 25–May 5, 2022, July 5–July 13, 2022, and September 28–October 5, 2022). S. alterniflora genetics, biomass, stem morphology, faunal community composition, and relevant abiotic conditions and site characteristics were measured at each site.
Genetic Diversity and Adaptive Potential in Marsh-Building Plants
To determine whether genotypic diversity differed between natural and restored marsh S. alterniflora populations and to assess the degree of genetic similarity among sites, S. alterniflora leaf clippings were taken in summer 2021 for genetic analysis. Clippings (n = 10 in a 1 m2 area) of a single leaf from 10 separate stems were taken immediately adjacent to each of the vegetation survey plots (n = 10, separated by ~ 4 m and described in more detail below) at each of the 12 marshes. Clippings were separately collected from leaves in plugs (1 leaf per plug) of S. alterniflora (n = 100) ordered from the 2 commercial nurseries most commonly used within the region for restoration plantings per discussions with restoration practitioner partners. These nurseries were located in New Jersey and Massachusetts. Clippings were preserved in silica until processing.
DNA was purified and extracted using standard methods (Hughes and Lotterhos 2014). Approximately 0.25 g of S. alterniflora leaf tissue was ground in a mill grinder (RETSCH MM 400, Verder International BV, Vleuten, Netherlands), and DNA was then extracted and purified using Omega Bio-Tek E-Z Plant DNA kit (Omega Bio-tek, Inc., Norcross, GA, USA). We used nine DNA microsatellite markers developed specifically for S. alterniflora to differentiate among genotypes (Blum et al. 2004; Sloop et al. 2005). PCR for all primers took place in 10 uL volume with the following reagents: 1 uL DNA template, 5 uL 2X type-it multiplex master mix (Qiagen, Venlo, Netherlands), 2 uL water, 0.25 uL each 10 mM forward primer for each of 4 loci, and 0.25 uL each 10 mM reverse primer for each of 4 loci. Forward primers were fluorescently labeled with NED, VIC, 6-FAM, and PET dyes. Loci were multiplexed for fragment length analysis. Using a T100 thermal cycler (Bio-Rad), PCR cycling conditions included initial activation/denaturation at 95 °C for 5 min, followed by 28 cycles of 95 °C for 30 s, 60 °C for 90 s, and 72 °C for 30 s, and final extension at 60 °C for 30 min. PCR products were separated on a 3730xl Genetic Analyzer (Applied Biosystems, Thermo Fisher Scientific, Waltham, MA, USA) with capillary electrophoresis at the Yale University DNA Analysis facility (New Haven, CT, USA) and fragment analysis was performed using GeneMarker version 2.6 (SoftGenetics). Clonal identity, diversity metrics, and genetic differentiation within and across sites were calculated in the program GENODIVE 3.06 (Meirmans 2020).
Structural Differences in Marsh-Building Plants
To determine whether plant morphology and growth characteristics differed between S. alterniflora in natural and restored marshes, S. alterniflora biomass and stem measurement sampling was conducted. At each of the 12 study sites, ten vegetation survey plots (0.25 m2) were established along the marsh edge in the S. alterniflora monoculture. Plots were positioned approximately 2 m from the creek bank and 4 m apart from each other. Transect locations within the marsh were chosen by reviewing aerial imagery in GIS (ArcGIS, Esri, Redlands, CA, USA) and comparing accessible marsh edge at all restored marshes. The smallest restored marsh edge was approximately 40 m in length, so that length was the maximum length established for the placement of plots within each marsh to ensure comparability among sites during analysis. In GIS, lines were drawn along the marsh edge for each site in an area where no major creeks had to be crossed to enable access by the sampling teams. Random points (n = 20) were generated along the line for each site at a minimum of 4 m apart. The points were each assigned a number from and one number was randomly selected for each marsh to be used as the starting point for plot placement (Supplemental Fig. 1). If the first randomly selected point was not suitable due to access or lack of S. alterniflora monoculture, the sampling team then moved to second randomized point (and so on) until suitable habitat was found for each site. Individual plot locations were then selected every 4 m from the starting point by haphazard quadrat toss along a meter tape parallel to the creek.
Measurements in each vegetation survey plot included stem density (per 0.25 m2 quadrat), flowering stem density (per 0.25 m2 quadrat), stem diameter (n = 10), and stem height (n = 10) during the 3 time points in 2021 for each plot. Stems measured for height and diameter were selected haphazardly and separately (i.e., 10 stems were haphazardly selected and measured for height and then 10 stems were haphazardly selected and measured for diameter). A sediment core (8 cm diameter, 20 cm depth) was collected adjacent to each plot (selected to be representative of growth within the plot) for above and belowground biomass analysis at the end of the fall 2021 sampling event. These biomass samples were transferred within 4 h to a −20 °C freezer, where they were held until analysis. For analysis, aboveground biomass was carefully clipped from core at the surface, gently washed to remove sediment over a 2-mm sieve, and separated into live and dead portions. Belowground biomass was divided into 2 depth sections (0–10 cm and 10–20 cm) for analysis. Each section was gently washed over a 2-mm sieve to remove sediment and debris. Care was taken to not break apart fine roots connected to other roots and rhizomes. After washing, the roots and rhizomes were sorted manually into live and dead portions using visual assessment. Each of the 6 portions was dried to a constant weight at 60 °C for 5 full days and weighed in grams.
To assess differences between natural and restored marshes in the plant community within and beyond the low marsh S. alterniflora zone, plant species identity and percent cover of all plants were quantified once at each study site during summer 2022 in each of the plots where faunal surveys were conducted (described below). Each survey plot location was located at 0 m, 3 m, 10 m, and 30 m from the water along transects perpendicular to the tidal creek or open water at 3 randomly selected intervals among the plant survey plots. At each of those 4 distance points on each of the 3 transects, triplicate plots were surveyed (n = 36 per marsh). In locations where the marsh transitioned to upland vegetation sooner than 30 m inland, then the 30 m plot was omitted. At each plot, the percent cover of all plant species was quantified in a 0.5 m2 quadrat through visual estimation. When unknown or cryptic species were encountered, photos or representative specimens were collected for review and identification offsite.
To determine the relative contributions of local environmental conditions and genetics in driving differences observed in S. alterniflora growth among sites and between marsh types, a common garden study was conducted at a natural marsh in Westport, CT, USA (not otherwise included in the group of natural marshes studied). Plant and sediment cores (8 cm diameter, 20 cm depth) were collected just to the side of each of 5 vegetation survey plots (every other) at all 12 marshes during the April/May 2021 sampling for transplant to the common garden. Core sample collection locations were biased to ensure S. alterniflora was present in the cores (i.e., cores were only collected over living stems, not areas of bare sediment without stems). Cores were transplanted to the common garden on the same day they were collected, and they were planted within 1–2 m of a tidal ditch or creek. Cores were wrapped in garden cloth and marked with an ID tag and flag to identify from which source marsh they came. Garden cloth was staked into the marsh so that core diameter remained intact and cores could be removed from the marsh at the end of the growing season for analysis. Stem density, stem diameter (of all stems present), and stem height (of all stems present) were collected at each of the individual cores during the three 2021 field sampling time periods. Additionally, 5 cores were taken from the common garden and transplanted to another location in the common garden. All transplanted cores (n = 65) as well as 5 natural common garden cores were removed from the common garden in October 2021 for above and belowground biomass analysis. Samples were transferred within 2 h to a −20 °C freezer and held until analysis.
Ecological Function
To determine differences in ecological function between natural and restored marshes as habitat, surveys of sessile and mobile animals were conducted in the spring, summer, and fall sampling events in both 2021 and 2022. Each faunal survey plot location was located at up to 4 distances from the water along 3 transects perpendicular to the tidal creek (0 m, 3 m, 10 m, and 30 m), with triplicate plots at each location as described above for plant species richness (n = 36 per marsh maximum). At each plot, all animals present and all crab burrows present were quantified within a 0.5 m2 quadrat. Nekton surveys were conducted in the summer sampling event of 2021 and 2022 through the deployment of un-baited Breder traps (n = 6) for 2 h encompassing high tide at each marsh during one high tide cycle (Breder 1960; Warren et al. 2002), after which all organisms present in the traps were identified and counted.
Site Characteristics
The following variables were measured in the study: grain size (n = 3 per marsh, May 2022); submergence frequency (n = 20 per marsh, July 2022); marsh surface elevation at each plot (n = 10 per marsh, September 2022); marsh edge slope (n = 10 per marsh, May 2022); plant species diversity (n = 10 per marsh, July 2022); temperature and salinity of tidal water (n = 1 per marsh per plant sampling date); soil temperature (n = 1 per marsh per sampling date); Spartina alterniflora end-of-season aboveground and belowground biomass (n = 10 per marsh, October 2021); Spartina alterniflora live stem height, density, diameter, and flowering stem density (n = 10 stems per plot, n = 10 plots per marsh, May, July, and September/October 2021); density of Geukensia demissa, crab burrows, and other marsh surface invertebrates (n = 12 per marsh, with n = 3 replicate plots at 0 m, 3 m, 10 m, and 30 m along a transect, May, July, and September/October 2021 and 2022); nekton abundance (n = 6 per marsh, in July 2021 and 2022); Spartina alterniflora genotype (n = 100 per marsh, with 10 leaves in each of 10 (ten) 1 m2 plots), July 2021; and n = 100 per greenhouse, n = 2 nurseries, July 2021). Approximate tidal range was determined based on the nearest NOAA tide gauge. These variables were sampled with different frequencies during these sampling events (Table S1).
To determine whether soil temperature differed between natural and restored marshes in the S. alterniflora zone, it was measured at each of the 12 study sites using a soil thermometer during each low tide sampling event in 2021 (spring, summer, and fall). To determine the relative contribution of water conditions in driving differences observed in S. alterniflora growth among sites and between marsh types, dissolved oxygen, water temperature, and salinity were measured using a YSI Pro2030 in the nearest primary creek at each of the 12 study sites and common garden during each of these same 3 sampling periods in 2021; water temperature and salinity were also measured during high tide in July once at each site in 2021 and 2022.
To determine whether differences in creek edge morphology between natural and restored sites exist that might impact the use of the marshes by nekton, marsh edge slope at the nearest tidal creek or open water to each vegetation survey plot was measured at all 10 plots at each of the 12 marshes and at the common garden in the spring sampling event of 2022. A meter tape with a weight attached to the end was fed through a PVC tube laid perpendicular to the marsh edge at each of the plots. The weighted end of the meter tape was lowered down to the sediment at distances of 0.5 m, 1 m, 1.5 m, and 2 m from the end of the vegetated marsh edge (Supplemental Fig. 2). Slope was calculated from the regression of a line through those points (from 0 to 2 m of distance from the marsh edge.
To determine whether sediment grain size differed among sites that might influence S. alterniflora growth or use of the marsh by marsh surface-dwelling animals, sediment samples (approximately 3 cm diameter, 10 cm depth) for grain size analysis were taken in the spring sampling event of 2022. A sediment core was collected beside 3 randomly selected vegetation survey plots (locations were intentionally selected within each area to minimize plant biomass in the core) in each of the 12 study sites and common garden. Samples were transferred within 4 h to a −20 °C freezer for preservation. Samples were shipped overnight to a contract laboratory (University of Connecticut, Center for Environmental Sciences and Engineering, Storrs, CT, USA) for analysis.
To determine the contribution of submergence frequency to differences observed among sites and between marsh types, marsh flooding was quantified at each study site and the common garden during 2022 (between June and August) using methodology adapted from prior studies (Smith and Warren 2007). Plastic plant garden stakes (n = 20) were painted with Elmer’s glue dyed with food coloring and allowed to dry. All stakes were then inserted into the marsh vertically in a haphazard arrangement around the 10 vegetation survey plots approximately 1–3 h before high tide. After one high tide, as soon as the water began to recede from the marsh, the height above the marsh surface of glue removed from each stake was measured in cm using a meter stick. The height of flooding on each stake was compared to local tide gauge records from the prior 12 months to determine flooding frequency (see detailed methods in Supplement).
Similarly, to determine the contribution of elevation to differences observed among sites and between marsh types, absolute elevation was measured during the fall 2022 sampling event at each of the 10 vegetation survey plots at each of the 12 study sites using a real-time kinematic positioning (RTK) Spectra SP85 GNSS Receiver (Spectra Geospatial, Trimble Inc., Sunnyvale, CA, USA) mounted to a 1.8-m tall tripod. These data were collected in addition to the submergence frequency calculation to enable an assessment of potential drivers of differences among sites where elevation and flooding frequency may be decoupled (e.g., sites experiencing tidal restriction due to infrastructure). The RTK receiver was connected via Bluetooth to a Carlson MINI2 Rugged Field Computer with SurvCE6 software (Carlson Software Inc., Maysville, KY, USA). An internet connection to the field computer was established using a Wi-Fi connection via a personal hot spot from an Apple iPhone. Once the field computer established a connection with the GPS network, measurements were taken. At each experimental plot, the tripod was placed directly in the center of the plot boundaries. The tripod was then adjusted until level. Using the field computer, 20 GPS position and elevation readings were taken and averaged for each plot. The mean position and elevation measurements were then recorded.
Data Analysis
Analysis of data was conducted in R version 4.2.0 and R Studio (R Core Team 2020; RStudio Team (2020)). Statistical method and any data transformations used are reported with the results for each analysis. When test assumptions were not able to be met using standard data transformations, nonparametric methods were employed. Comparisons made at the “site level” refer to a measured or aggregate metric for a given study site (e.g., all plots at one marsh), whereas comparisons made at the “plot” level refer to a measured or aggregate metric within a specific plot location (of which there are multiple per site, or marsh).
Comparisons of Groups
Analysis of variance (ANOVA) was used to assess differences between restored and natural marshes for soil temperature, water temperature, dissolved oxygen, and salinity, grain size, stem height, density, and diameter, flowering stem density, plant species composition and cover, plant species richness, change-with-transplantation over time in above and belowground biomass, transplantation effects on height in the common garden, differences in flooding and salinity in the common garden, transplantation effects on stem density and diameter in the common garden. Student’s t-test was used to assess differences between restored and natural marshes for the effective number of genotypes and allelic richness. Tukey’s honestly significant difference test was used to test for pairwise differences in salinity between each pair of sites. Kruskal–Wallis chi-squared test was used to assess differences between restored and natural marshes for marsh edge slope, flooding frequency, elevation, live and dead above and belowground biomass, genetic differentiation (Fst) among site pairs, nekton species richness, fish species richness, nekton abundance, and fish abundance. Kruskal–Wallis rank sum test was used to assess differences between restored and natural marshes for abundance of common marsh nekton species (Palaemonetes pugio, Fundulus heteroclitus, Cyprinodon variegatus, Menidia menidia). Fisher’s exact probability test was used to test for the difference between restored and natural marshes in the likelihood of plants from each site showing a change at the common garden across all biomass parameters (0–20 cm live, dead, and total belowground biomass, and live, dead, and total aboveground biomass).
Linear Relationships
Linear regression was used to test for a relationship between the flooding frequency and elevation data for each site, genetic differentiation with increasing distance (i.e., isolation by distance) for the natural marshes, the restored marshes, and for all marshes (with site pairs greater than 40 km apart excluded), live belowground biomass and the change in salinity and flooding in the common garden, live belowground biomass and the effective number of genotypes in the common garden, S. alterniflora genotypic diversity (effective number of genotypes) and live stem density, flowering stem density, stem height, or stem diameter, for a relationship between genetic differentiation and proximity between natural and restored sites, and for a relationship between S. alterniflora biomass and time since restoration (for restored sites). Multiple linear regression was used to assess differences in the increase in height during the growing season from the field to the common garden with the differences in flooding and salinity between the field and the common garden, effective number of genotypes, and corrected Shannon diversity index, for natural and restored marshes. A multiple linear model was used to assess the relationship between effective number of genotypes and site type (natural or restored) at the plot level and at the site level with fall live stem density, flowering stem density, stem height, and stem diameter. Kendall-Theil Sen Siegal nonparametric linear regression was used to test for a relationship between genetic differentiation between a given restored site and the natural sites with time since restoration, and flowering stem density and the effective number of genotypes.
Other Analyses
Scheirer-Ray-Hare test was used to assess differences in elevation change with increasing distance inland within the marsh between restored and natural marshes. Principal component analysis was used to assess the key drivers of structural and functional differences between the restored and natural marshes, incorporating both abiotic and biotic variables.
Results
Genetic Diversity and Adaptive Potential in Marsh-Building Plants
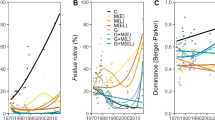
Across the 12 marshes studied, the mean effective number of genotypes of S. alterniflora was lower in natural (5.308 ± 1.352) than restored (24.30 ± 4.450) sites (Student’s t-test, rank-transformed, p = 1.83e−3), as was allelic richness in natural (13.67 ± 3.480) than restored (40.67 ± 3.703) sites (Student’s t-test, rank-transformed, p = 3.4e−4). There was no evidence of genetic isolation by distance in S. alterniflora when all 12 sites were considered together (Fig. 1; linear regression, rank-transformed; F (1, 142) = 2.023, p = 0.1571), or when considering distance between each restored site and the 6 natural sites (linear regression, rank-transformed; F (1, 34) = 2.472, p = 0.1252). However, isolation by distance was observed for S. alterniflora within the natural marshes only (linear regression, rank-transformed; F (1, 34) = 4.385, p = 4.378e−2), and within the restored marshes only (linear regression, rank-transformed; F (1, 34) = 5.398, p = 2.6e−2). Isolation by distance was also observed for S. alterniflora when site pairs greater than 40 km apart were excluded from the analysis (linear regression, rank-transformed; F (1, 62) = 24.48, p < 0.0001).
(left) Genetic differentiation (Fst) among Spartina alterniflora from different site pairs (n = 6 natural and n = 6 restored) with increasing distance between sites. (right) Genetic differentiation (Fst) among Spartina alterniflora from different site types (natural salt marsh, restored salt marsh, and commercial nursery)
The natural marsh populations of S. alterniflora were less closely related to each other (as measured by Fst, a metric of differentiation among populations) than they were to the restored sites (Fig. 1; Kruskal–Wallis Χ2 = 51.36, df = 5, p < 0.0001). S. alterniflora plugs purchased from two commercial plant nurseries for this study (as they are nurseries known to regularly supply local restoration projects) were found to be closely related to each other. The restored salt marsh plants were more closely related to the nursery plants than they were to the natural marsh plants (Fig. 1). Differentiation between a given restored site and the natural sites decreased with time since restoration (Kendall–Theil Sen Siegel nonparametric linear regression, p = 3.08e−4); however, the limited number of restored marshes included in the study, and the relatively short time since their restoration activities, may limit the broader applicability of this finding.
Structural Differences in Marsh-Building Plants
No difference was observed between natural (2490.8 ± 144.2 g m−2) and restored (2331.1 ± 149.8 g m−2) marshes in S. alterniflora live belowground biomass in the top 20 cm (Kruskal-Wallace, p = 4852). However, natural marshes (4853.4 ± 184.0 g m−2) had 223.5% more S. alterniflora dead belowground biomass than restored marshes (1500.2 ± 305.2 g m−2) in the top 20 cm (Fig. 2; Kruskal–Wallis Χ2 = 49.56, df = 1, p < 0.0001). Restored marshes had more S. alterniflora live aboveground biomass than natural marshes (Kruskal–Wallis Χ2 = 25.50, df = 1, p < 0.0001). No difference was observed between natural and restored sites in S. alterniflora dead aboveground biomass (Kruskal-Wallace, p = 0.4434). At the restored sites, no relationship was observed between increasing time since restoration (i.e., marsh age) and belowground biomass (linear regression, rank-transformed for all, 0–10 cm dead: F (1, 58) = 0.322, p = 0.573; 10–20 cm dead: F (1, 58) = 1.788, p = 0.186; 0–10 cm live: F (1, 58) = 0.148, p = 0.702; 10–20 cm live: F (1, 58) = 0.002, p = 0.969). No relationship was observed between increasing time since restoration and dead aboveground biomass (F (1, 58) = 0.221, p = 0.64), but live aboveground biomass decreased with time (F (1, 58) = 6.091, p = 0.0166).
Belowground Spartina alterniflora biomass in the field and common garden from natural and restored salt marshes. Letters indicate significant differences (ANOVA, Tukey HSD, p < 0.05) between mean natural, restored, field, and common garden values within each group of bars for the categories shown along the x-axis (letters are not comparable among different depths and live or dead status groups)
Across the 3 time points during the growing season (spring, summer, and fall), the restored marsh S. alterniflora had a greater stem height, greater stem diameter, and lower stem density than in the natural marshes (ANOVA, rank-transformed; p < 0.001 for all; Supplemental Figs. 3–5). S. alterniflora flowering stem density was higher in restored (3.033 ± 0.383 per 0.25 m2) than in natural marshes (2.033 ± 0.380 per 0.25 m2) during the fall (ANOVA, F (1, 118) = 7.444, p = 7.34e−3; rank-transformed), which was the only survey during which flowering stems were observed. S. alterniflora flowering stem density was not related to the effective number of genotypes within that plot (Kendall-Theil Sen Siegel nonparametric linear regression, p = 0.3370).
Restored sites were found to have lower percent cover of S. alterniflora (ANOVA, rank-transformed; F (1, 108) = 4.006p = 4.784e−2) and higher percent cover of bare space (ANOVA; F (1, 108) = 16.83, p < 0.0001) than natural marshes (Supplemental Fig. 6). Natural marshes had a higher percent cover of Spartina patens (ANOVA, rank-transformed; F (1, 108) = 16.87, p < 0.0001) and Distichilis spicata (ANOVA, rank-transformed; F (1, 108) = 6.999, p = 9.373e−3). Plant species richness at plots 0 m, 3 m, and 10 m from the marsh edge (pooled) was greater at natural than at restored sites (ANOVA, rank-transformed; F (1, 108) = 10.70, p = 0.0012; Supplemental Fig. 7).
In the common garden study, no difference in change-with-transplantation over time was observed between restored and natural sites for plant biomass (ANOVA, live 0–20 cm: F (1, 10) = 1.309, p = 0.2790; dead 0–20 cm: F (1, 10) = 1.007, p = 0.3390, live aboveground biomass: F (1, 10) = 3.111, p = 0.1080, or live aboveground to belowground allocation ratio: F (1, 10) = 4.587, p = 5.790e−2). Thus, S. alterniflora in restored and natural marshes responded similarly to transplantation to the common garden when considering all sites pooled. Belowground biomass patterns between S. alterniflora in restored and natural marshes were largely retained in the common garden. No relationship was observed between S. alterniflora live belowground biomass and the change in salinity (between each source site and common garden; F (3, 8) = 0.4280, p = 0.7385; t = 0.143, p = 0.8895), the change in flooding frequency (between each source site and common garden; t = 8.9e−2, p = 9316), or the effective number of genotypes (t = 0.911, p = 0.3889). Thus, restored sites and natural sites were similarly likely to have shown a change (on an individual site basis) at the common garden across all S. alterniflora biomass parameters considered (Fisher’s exact probability test, 0–20 cm live: p = 0.1818, 0–20 cm dead: p = 1.0, and total: p = 6.061e−2 belowground biomass, and live: p = 0.4545, dead: p = 1.0, and total: p = 0.5455 aboveground biomass;). That is, restoration status (restored or natural) did not impact change-with-transplantation in S. alterniflora biomass at the common garden.
Changes in S. alterniflora height between the field and common garden were largely explained by corresponding changes in salinity and flooding frequency between each field site and the common garden’s conditions. Increasing flooding (p = 1.2e−2) and decreasing salinity (p = 1.81 e−3) were associated with greater increases in height during the growing season between the field to the common garden (multiple linear regression, R2 = 0.74, F = 16.37, p = 1.003e−3). However, transplantation effects on height were also different between the restored and natural sites when considered alone without other factors (ANOVA, F = 6.907, p = 2.52e−2). The differences in flooding (ANOVA, F (1, 10) = 1.751, p = 0.2150) and salinity (ANOVA, F (1, 10) = 1.995, p = 0.1880) between restored and natural marshes were similar. However, when incorporated as a factor into the multiple linear regression model, the overall model remained significant (F (3, 8) = 12.65, p = 2.095e−3), but only driven by the flooding (t = 2.544, p = 3.45e−2) and salinity (t = −3.666, p = 6.35e−3); site type was not a significant factor in driving these changes (t = −1.382, p = 0.2044). Neither effective number of genotypes (t = −0.5630, p = 0.5891) nor corrected Shannon diversity index were related to the observed changes in height (t = −0.556, p = 0.5935) when incorporated into the multiple linear regression model. Unlike with stem height, transplantation effects on stem density (ANOVA, F (1) = 0.2680, p = 0.6160) and diameter (ANOVA, F(1) = 0.0010, p = 0.9770) were not related to whether the site was natural or restored (see Supplement for additional results from the common garden study).
Benthic Invertebrates and Nekton
Abundance at 0 m and 3 m from the marsh edge was higher in natural marshes than restored marshes for Geukensia demissa and burrowing crabs (0 m only), but not for Melampus bidentatus (Table 2). On an individual site basis, invertebrate abundance at some restored sites was found to be higher than some of the natural marshes, with similar abundances of some or all of those species observed between specific individual natural and restored sites (Fig. 3).
Unlike the differences observed in marsh-platform resident species, no difference was observed in nekton species richness between natural (2.07 ± 0.199 species per trap) and restored (2.17 ± 0.370 species per trap) marshes (Kruskal–Wallis rank sum test: Χ2 = 0.3152, df = 1, p = 0.5745) nor when only fishes were considered alone for natural (1.44 ± 0.113 species per trap) and restored (1.35 ± 0.278 species per trap) marshes (Kruskal–Wallis rank sum test: Χ2 = 0.1637, df = 1, p = 0.6858). Mean nekton abundance (across all species) did not differ between natural (10.4 ± 3.05 individuals per trap) and restored (18.5 ± 5.78 individuals per trap) marshes (Kruskal–Wallis rank sum test: Χ2 = 0.4103, df = 1, p = 0.5218), nor when only fishes were considered alone (natural 5.97 ± 1.29 fish per trap; restored 6.85 ± 2.31 fish per trap; Kruskal–Wallis rank sum test: Χ2 = 0.1608, df = 1, p = 0.6884). Additionally, no difference was observed between restored and natural marshes for the abundance of Palaemonetes pugio (Kruskal–Wallis rank sum test, Χ2 = 1.775, df = 1, p = 0.1828), Fundulus heteroclitus (ANOVA, F (1, 22) = 0.8872, p = 0.3565), Cyprinodon variegatus (Kruskal–Wallis rank sum test, Χ2 = 8.461e−2, df = 1, p = 0.7712), or Menidia menidia (Kruskal–Wallis rank sum test, Χ2 = 0.3496, df = 1, p = 0.5543).
Site Characteristics
There were few consistent differences between the restored and natural sites in the abiotic parameters measured (raw and summary data can be found in Supplemental Tables 2–5). No differences were observed between restored and natural salt marshes in soil temperature at any of the individual sampling events in 2021 (ANOVA, spring: F (1, 10) = 0.7760, p = 0.3990; summer: F (1, 10) = 4.7e−2, p = 0.8320; and fall: F (1, 10) = 1.0e−2, p = 0.9210) or in mean soil temperature (ANOVA, F (1, 10) = 0.4390, p = 0.5230). Higher dissolved oxygen was only observed in the water at low tide in the spring 2021 sampling event at the restored marshes (ANOVA, F (1, 10) = 11.36, p = 7.12e−3), but no differences were observed between marsh types in the summer (ANOVA, F (1, 9) = 0.18, p = 0.6810) or fall sampling events (ANOVA, F (1, 8) = 1.251, p = 0.296). A difference was observed in water temperature between restored and natural sites during the summer sampling event at high tide in 2021 (ANOVA, F (1, 10) = 7.197, p = 2.3e−2), but not in 2022 (ANOVA, F (1, 10) = 0.2140, p = 0.6530), nor during the low tide sampling events in the spring (ANOVA, F (1, 10) = 2.849, p = 0.1220), summer (ANOVA, rank-transformed, F (1, 10) = 0.1480, p = 0.7080), or fall (ANOVA, F (1, 10) = 2.000e−3, p = 0.9660) of 2021.
No difference in mean salinity between restored and natural salt marshes was observed during the low tide sampling events in spring (ANOVA, F (1, 10) = 1.989, p = 0.1890), summer (ANOVA, F (1, 10) = 1.037, p = 0.3330), or fall (ANOVA, rank-transformed, F (1, 10) = 0, p = 1.0) in 2021, nor the high tide sampling events in 2022 (ANOVA, rank-transformed, F (1, 10) = 2.328, p = 0.1580). When aggregating salinity data from all 4 of these sampling events, there was an effect of both site type (restored or natural; two-way ANOVA, F (1, 45) = 4.271, p = 0.04456) and tide (low or high; two-way ANOVA, F (1, 45) = 11.674, p = 0.00135) on observed salinity. However, analysis of the data by site revealed differences among sites (ANOVA, F (11, 36) = 5.483, p < 0.0001) that appear to be driven by a single outlier in the restored marsh group (Moses Wheeler, Milford, CT) that consistently had a lower salinity than 9 out of 11 of the other sites (Tukey HSD, Branford p = 2.913e−2, Calf p = 3.098e−2, Farm p = 1.434e−3, Groton Airport p = 1.429e−2, Groton Long Point p < 0.0001, Niantic p = 1.482e−4, Oyster p = 0.5475, Pattagansett p = 1.047e−4, Sikorsky p = 9.49e−3, Stratford p = 3.146e−2, West p = 0.3656).
The downward marsh edge slope from the vegetated marsh to the water was found to be greater (i.e., steeper) at the natural marshes (−0.242 ± 0.0282) than at the restored (−0.168 ± 0.0275) marshes (Kruskal–Wallis Χ2 = 16.94, df = 1, p < 0.0001; Supplemental Table 6).
Within each of the 3 sediment grain size fractions considered (sand, silt, and clay), there were no differences in the fraction percentage between natural and restored marshes (ANOVA, clay: F (2, 36) = 0.4673, p = 0.6304; silt: F (2, 36) = 0.6185, p = 0.5444; sand: F (2, 36) = 0.7292, p = 0.4893). Site-specific interpretations were limited due to the small number of replicates collected per marsh for this analysis (Supplemental Table 7).
Submergence frequency (as measured using the tide stick method) at the restored marsh vegetation survey sampling plots (31.28 ± 16.45%) was higher than at natural (20.09 ± 12.72%) marshes (Kruskal–Wallis Χ2 = 13.59, df = 1, p = 2.271e−4; Supplemental Table 8). As expected, plot elevation at those same locations (as measured using the RTK unit) was also higher at the natural than at the restored marshes (Kruskal–Wallis Χ2 = 23.79, df = 1, p < 0.0001). Importantly, both observed flooding and elevation were considered due to the potential for some of the marshes to be experiencing tidal restriction, such that their elevation might not be representative of the duration of submergence as would be expected from their elevation alone. However, the two methods were found to be in close agreement for the 12 sites (R2 = 0.82) when the one site known to experience tidal restriction (Sikorsky Airport) was omitted.
The positioning of the marsh surface fauna plots along transects running upland perpendicular to the water provided an opportunity to consider differences in elevation change (e.g., slope) across the marsh platform between restored and natural sites (beyond the area captured in the vegetation plot analyses). The difference in mean elevation between natural and restored marshes appears to decrease moving upland onto the marsh platform; elevation was only different at locations in the marsh closest to the creek edge when each distance was considered individually (pairwise t-test, p = 0.049). However, a multivariate analysis revealed an effect of both distance upland (Scheirer Ray Hare test; H (2, 89) = 10.34, p = 5.68e−3) and restored or natural site type (Scheirer Ray Hare test; H (1, 89) = 6.058, p = 1.384e−2) on elevation.
Interactions Between Structure, Function, and Genetics
When considered at the site scale (i.e., pooling plots within a site), no relationship was observed between S. alterniflora genotypic diversity (effective number of genotypes) and fall 2021 live stem density (linear regression, rank-transformed, F (1, 10) = 1.969, p = 0.1908), flowering stem density (linear regression, rank-transformed, F (1, 10) = 0.5272, p = 0.4845), stem height (linear regression, F (1, 10) = 3.759, p = 8.125e−2), or stem diameter (linear regression, F (1, 10) = 2.973, p = 0.1154). However, when considered at the individual plot scale (i.e., averaging stem values within each plot within a site), two relationships were observed: lower fall live stem density (Kendall–Theil Sen Siegel nonparametric linear regression, p < 0.0001) and taller fall stem height (Kendall–Theil Sen Siegel nonparametric linear regression, p = 1.14e−3) with increasing effective number of genotypes. A multiple linear model including both effective number of genotypes and site type (natural or restored) revealed no relationships again at the site level but did reveal relationships at the plot level with fall live stem density (F (2, 113) = 14.4, p < 0.0001), flowering stem density (F (2,113) = 3.22, p = 4.365e−2), stem height (F (2, 113) = 14.8, p < 0.0001), and stem diameter (F (2, 113) = 11.83, p < 0.0001).
A principal component analysis revealed overlapping clustering by marsh type (restored or natural) (Fig. 4), with the first 3 principal components accounting for 31.1%, 18.8%, and 15.3% of the total variance. The first principal component was driven largely by parameters related to plant biomass and fauna (0 m faunal richness, stem height, stem diameter, effective number of genotypes, live aboveground biomass, average effective number of genotypes per plot, dead belowground biomass, nekton count, and others). The second principal component was driven largely by site elevation, live belowground biomass, G. demissa density at the marsh edge, nekton species richness, and whether the site studied was in the eastern Long Island Sound or western Long Island Sound. The third principal component was driven largely by grain size, edge slope, M. bidentatus density, and crab burrow density. This analysis enabled us to assess the key drivers of structural and functional differences between the restored and natural marshes. While three restored sites (Groton Airport, restored in 2012; Moses Wheeler, in 2016; and Niantic, in 2017) appeared separated from the natural marsh cluster, this analysis did not suggest a consistent difference between restored and natural marshes when considering variables in aggregate.
Principal component analysis incorporating plant, animal, and genetics parameters. The third principal component (not shown) accounted for 15.3% of the total variance. The first principal component was driven largely by parameters related to plant biomass and fauna (0 m faunal richness, stem height, stem diameter, effective number of genotypes, live aboveground biomass, average effective number of genotypes per plot, dead belowground biomass, nekton count, and others). The second principal component was driven largely by site elevation, live belowground biomass, G. demissa density at the marsh edge, nekton species richness, and whether the site studied was in the eastern Long Island Sound or western Long Island Sound. The third principal component was driven largely by grain size, edge slope, M. bidentatus density, and crab burrow density
Discussion
Genetic Diversity and Adaptive Potential in Marsh-Building Plants
The greater S. alterniflora genetic diversity in actively planted restored marshes may increase resilience by providing a larger pool of potentially well-suited genotypes that may confer a fitness advantage as abiotic conditions change more rapidly with climate change. Intraspecific trait variation driven by genotype has been shown to result in rapid evolution in marsh plant allocation and distribution of belowground biomass (Vahsen et al. 2023). As much as 49.5% of belowground biomass variation (and resultant marsh elevation gain) was found to be explained by genotype in another marsh ecosystem engineer, Schoenoplectus americanus (Vahsen et al. 2023). With increasing acknowledgement of the importance of genotype and intraspecific genetic diversity on ecosystem dynamics (Vahsen et al. 2023), rapid trait evolution may enable these restored marshes’ plant populations to respond on timescales relevant to the pace of anthropogenic climate change due to their greater adaptive potential. Alternatively, given the genetic differentiation of restored sites versus natural sites, this higher diversity may not be associated with an increase in locally beneficial traits. The role of genetic diversity in driving resilience has also been demonstrated in other plant-based ecosystems such as forests (Nef et al. 2021) and seagrasses (Williams 2001; Ehlers et al. 2008; Plaisted et al. 2020), and is broadly considered an important driver of ecosystem resilience under climate change (Bernhardt and Leslie 2013; Timpane-Padgham et al. 2017).
There has long been interest in using local genotypes for restoration efforts (Jones 2003), but our understanding of how to select plant sources and genotypes for restoration to promote resilience with climate change is still developing (Adam 2019). For example, a recent study of marshes in Jamaica Bay, NY, recommended that S. alterniflora used for restoration should be sourced from within the same region as the area being restored, but not necessarily from within the same marsh. However, their definition of “region” was very geographically broad (e.g., New England or North Mid-Atlantic), providing limited guidance for restoration practitioners designing for sites in other areas. Importantly though, their work suggests that biogeographic breaks for S. alterniflora should be considered (Blum et al. 2007; Novy et al. 2008). S. alterniflora has been shown to have high levels of small-scale genetic diversity, especially for a clonal species (Richards et al. 2004; Hughes and Lotterhos 2014; Foust et al. 2016; Robertson et al. 2017). Though recent work in a mid-Atlantic salt marsh found that S. alterniflora populations were dominated by few, well-adapted clones, with sexual reproduction and recruitment from other sites providing avenues to increase diversity and resulting resilience (Walker et al. 2021). Thus, restoration practitioners need to consider not only the location of the source population for purchased plugs when sourcing plants for restoration, but also how those plants will contribute to resilience either through their phenotypic characteristics, their genotypic diversity, and the relatedness of those plants to those that might colonize the site naturally over time.
Our finding that the restored sites were not more closely related to the natural sites closest to them suggests that the restored site S. alterniflora populations were not being significantly influenced by natural seeds arriving through dispersal, at least on the timescale post-restoration studied here (4–9 years). Thus, the initial pool of genotypes introduced to a given site during restoration planting may play a key role in determining the genetic composition of the S. alterniflora population for a prolonged period of time. This was also evidenced by the genetic differentiation between the plants from the restored sites and commercial nurseries being lower than was observed between the plants from the restored and natural sites. Our observation of increasing time since restoration leading to more similarity in S. alterniflora in the restored marshes with the natural sites could be the result of gene flow, anthropogenic stressors, or other factors. However, the limited number of restored sites studied here, and only one of those having been restored more than 6 years prior to the study, potentially limits the broader applicability of this finding. As additional time passes since restoration, selective pressures on transplant survival, seed production, and seed viability, along with more rapid clonal expansion of fitter genotypes, may alter these patterns.
The method by which revegetation is accomplished during marsh restoration has meaningful implications for the genetic diversity of the restored site, and how it compares to natural populations. In the present study, the six restored marshes were planted with plugs of S. alterniflora purchased from commercial nurseries. The higher levels of genotypic diversity observed in our commercial nursery samples (nearly all individuals sampled were unique) relative to the field sites indicate that planting of plugs from these sources is likely to increase diversity at a local scale relative to nearby marshes. The close relation between the two nurseries studied could potentially suggest that one purchased source plants from the other or they used a similar field source site, which could also help to explain the relatedness within the restored sites as there are a relatively limited number of commercial nurseries that offer this species for sale. These restoration projects may thus result in a genetic structure of the restored marsh plants that differs from nearby natural marshes, and this may be especially relevant when restored with genotypes from populations that are not local (Kettenring et al. 2014). Our data suggest that each plant sent from two nurseries sampled was all grown from seed rather than from clonal propagation, whereas natural marshes are populated by plants through both clonal and non-clonal establishment.
However, in some restoration projects, revegetation is accomplished through planting transplants collected from natural marshes or allowing natural colonization by seed or even by episodic rafting of peat and plants over time. In these types of restoration projects, the resultant genetic diversity has been found to be similar between restored and natural sites (Gaynor et al. 2019). Similar diversity between natural and restored marsh plants has also been observed in restored areas allowed to revegetate naturally (Travis et al. 2002). When collecting local plants from the field for restoration efforts, some researchers have advocated for propagation from locally collected seeds (rather than transplanting plug directly) to maximize diversity in restoration plantings (Novy et al. 2010). Thus, the mechanism by which the plants are sourced (including the propagation practices of individual commercial vendors) can impact the resultant diversity and adaptive potential and may drive differences in resilience among restored sites (Ehlers et al. 2008; Schaberg et al. 2008; Reynolds et al. 2012; Kettenring et al. 2014; Aavik and Helm 2018; Nef et al. 2021).
Structural Differences in Marsh-Building Plants
As it is an important contributor to elevation gain, lower accumulation of dead belowground biomass in restored salt marshes may make them less resilient to increasing rates of sea-level rise. Plants contribute to salt marsh elevation in multiple ways, including retention of sediment, accumulation of organic matter, and prevention of erosion (Cahoon et al. 2021). The genetics of S. alterniflora used in restoration may be especially important due to the differing mechanisms by which marshes keep pace with sea-level rise. In marshes along the Southern Atlantic and Gulf coasts of the USA, many marshes are more sediment-based, relying largely on robust aboveground biomass to capture suspended sediment and accumulate that sediment over time to build elevation. In the more sediment-starved Northeastern US, salt marshes tend to be more peat-based, relying on the build-up of plant belowground biomass for the maintenance of elevation. The extent to which the differences in S. alterniflora growth form related to biomass allocation and other factors are driven by plasticity or the existence of ecotypes has been debated extensively (Shea et al. 1975; Valiela et al. 1978; Gallagher et al. 1988) (and likely varies across sites), but biogeographic trends and genetic differences are evident (Blum et al. 2007; Kirwan et al. 2009; Crosby et al. 2017). These spatial differences in S. alterniflora point to the need for appropriately considering source population in restoration. Sediment-starved salt marshes will require adequate belowground biomass accumulation to withstand sea-level rise, especially as suspended sediment concentrations decline (Weston 2014), and eutrophic conditions drive creek bank collapse and belowground decomposition (Deegan et al. 2012; Watson et al. 2014).
Differences in biomass have been observed in some comparative studies of restored and natural marshes (Rezek et al. 2017; Wang et al. 2021; Liu et al. 2024). The common garden findings here suggest that the observed differences in morphology were related to marsh type (restored or natural) and are thought to be constitutive. However, since the transplantation was done in adult plants, it is possible that the study design may not have captured the developmental window during which a plant response was possible. If retained, these differences can impact productivity, carbon sequestration, wave attenuation, and resilience.
With the restored marshes studied being relatively young (4–9 years since planting), it is important to consider the impact of marsh age on the differences observed. Previous work comparing restored marshes over time has found that above and belowground biomass may change with marsh age relative to natural marshes (Craft et al. 1999). Craft and colleagues (1999) found that in two restored marshes, aboveground biomass was similar to or even exceeded that in paired natural marshes within the first two to three growing seasons after restoration. Similar to the within-marsh temporal patterns observed in those sites, live aboveground biomass was higher at the youngest restored marshes studied here, and decreased with increasing age. However, abiotic differences among sites within our dataset may complicate those relationships. Belowground, Craft and colleagues found that the restored salt marshes had similar biomass to that of natural marshes only 3 to 5 years after restoration, and belowground biomass continued to increase in subsequent years. Unlike in those marshes, 4–9 years was not sufficient for the restored salt marshes to achieve similarity to natural marshes for some metrics, most notably dead belowground biomass, nor was there an increase in biomass with time. However, this difference could be due to the geographic and climatological differences between that study and the present study; in North Carolina, total belowground biomass may be relatively less influenced by dead belowground biomass than in the more northern marshes studied here due to latitudinal differences in biomass allocation and decomposition rates (Crosby et al. 2017). Since biomass in restored salt marshes can be highly dynamic in the years following restoration (Craft et al. 1999), we might expect the observed differences between restored and natural marshes to continue to evolve and may progress differently among sites.
The higher density of flowering stems in restored marshes could potentially be related to the higher genotypic diversity of the S. alterniflora population and the relatively lower proportion of individual stems that are clonally related. Importantly, though, since S. alterniflora in the restored marshes is producing more flowers and potentially more viable seeds due to the reduced effects of inbreeding on seed viability at higher diversity populations, restored marsh plant populations might have a disproportionate impact on the local gene pool and colonization dynamics of surrounding sites. Additionally, if these more genetically diverse marsh plant populations are producing more seeds, they might also be able to sustain their own high levels of genetic diversity into the future.
Benthic Invertebrates and Nekton
Despite the differences observed in plant structure and genetics, the ecological function of the restored marshes to provide habitat for nekton was found to be similar to natural marshes. However, the lower density of key benthic invertebrates known to directly and indirectly interact with marsh plants may result in less resilient restored marshes. This was consistent with prior research that has found similar macrofauna assemblages in as few as 4–5 years after restoration of an S. alterniflora marsh (Warren et al. 2002; Rezek et al. 2017). However, use by several of the marsh-resident faunal engineers differed, such as crabs, snails, and mussels. While some species are able to colonize quickly, previous work has shown that establishment of benthic infauna at a restored site can take a longer period of time post-restoration than was studied here: 25 years or more in some instances (Craft and Sacco 2003). Increased genotypic diversity of foundation species has also been shown to increase the diversity of associated organisms (Whitham et al. 2003; Crutsinger et al. 2006). Thus, though animal use at the restored marshes was lower than at natural sites, the increased genotypic diversity of the S. alterniflora in the restored marshes suggests that they could support higher diversity faunal assemblages once those species disperse to and establish at those sites.
The lower abundance of Geukensia demissa in restored salt marshes not only indicates lower functionality of the marsh in terms of water quality and nutrient processing, but may also make these marshes less able to keep pace with sea-level rise. G. demissa pseudofecal biodeposits have been shown to not only stimulate S. alterniflora on local scales by increasing nutrient availability (Bertness 1984; Derksen-Hooijberg et al. 2019), but also directly contribute to salt marsh accretion (Crotty et al. 2023). These mussel biodeposits contribute to accretion in the immediate area where the mussels are present, but also are distributed rapidly and be retained, at least in part, locally within the marsh (Crotty et al. 2023). Beyond the structural and functional role of the mussels alone, the co-occurrence of S. alterniflora and G. demissa has been shown to enhance other ecosystem functions such as primary productivity, decomposition, species richness of other organisms (Angelini et al. 2015), and disturbance recovery (Derksen-Hooijberg et al. 2019). As such, the higher abundance of mussels at the natural marshes in the present study may provide an accretion rate benefit relative to the restored marshes to help them keep pace with sea level, while also increasing multifunctionality. However, this benefit may return in restored marshes with time, as others have found that the density of G. demissa increases with restored marsh age, and that the difference in G. demissa density may be due to lower larval survival in restored areas, rather than larval supply or settlement (Bilkovic et al. 2021).
However, in eutrophic systems where nitrogen may not be limiting, the reduction in belowground biomass allocation driven by high nitrogen availability both increases erosion susceptibility and decreases elevation gain, such that the nutrient transfer to the plants from the mussels is no longer providing a growth and accretion benefit (Whaley and Alldred 2023). The effect of this mutualism has also been shown to be lower in restored or constructed salt marshes relative to natural marshes, suggesting potential limitations on the utility of incorporating mussels into future restoration design (Whaley and Alldred 2023). Furthermore, a study of the interactive effects of S. alterniflora, G. demissa, and Uca pugnax found a reduction in S. alterniflora growth and disturbance recovery with the greater crab abundance associated with mussel aggregations through a negative feedback loop (Derksen-Hooijberg et al. 2019). As such, the relative importance of benthic invertebrates in restored and natural marsh resilience may depend on seascape context, interspecific interactions, and local abiotic conditions.
Implications for Restoration
Restored salt marshes may differ in their response to warming and sea-level rise from natural marshes, thus impacting their potential for similar long-term function. With greater belowground biomass, abundance of ribbed mussels, and higher current elevations observed at the natural marshes studied, these restored marshes may be at relatively greater risk of drowning under sea-level rise. Notably, however, the restored salt marshes studied achieved similar mobile animal habitat function in only 4–9 years, indicating that even on short time scales, these restored sites may be playing important ecological roles. Managers should focus on how to both restore function and achieve resilience as quickly as possible. The more genetically diverse plant populations that have resulted from restoration plantings may provide a mechanism for these marshes to adapt, but the long-term implications of these differences under climate change are unknown particularly given the genetic differentiation among restored and natural sites. Greater collaboration among restoration practitioners and the commercial nurseries from whom they are sourcing plants may be important to ensure that the provenance of these sources is appropriate for the sites in which they are being planted. Additionally, consideration should be given to the co-restoration of multiple species in addition to S. alterniflora and species like ribbed mussels, along with other novel engineering techniques that may be able to provide ecosystem engineering services more quickly than in a natural settlement scenario (Temmink et al. 2023).
Data, Material, and/or Code Availability
Data will be made publicly available at the time of publication via DataDryad.org (https://doi.org/10.5061/dryad.rjdfn2zmd).
References
Aavik, T., and A. Helm. 2018. Restoration of plant species and genetic diversity depends on landscape-scale dispersal. Restoration Ecology 26: S92–S102. https://doi.org/10.1111/rec.12634.
Adam, P. 2019. Chapter 23 - salt marsh restoration. In Coastal Wetlands (Second Edition), ed. G.M.E. Perillo, E. Wolanski, D.R. Cahoon, and C.S. Hopkinson, 817–861. Elsevier. https://doi.org/10.1016/B978-0-444-63893-9.00023-X.
Angelini, C., T. van der Heide, J.N. Griffin, J.P. Morton, M. Derksen-Hooijberg, L.P.M. Lamers, A.J.P. Smolders, and B.R. Silliman. 2015. Foundation species’ overlap enhances biodiversity and multifunctionality from the patch to landscape scale in southeastern United States salt marshes. Proceedings of the Royal Society B: Biological Sciences 282. Royal Society: 20150421. https://doi.org/10.1098/rspb.2015.0421.
Barbier, E.B., S.D. Hacker, C. Kennedy, E.W. Koch, A.C. Stier, and B.R. Silliman. 2011. The value of estuarine and coastal ecosystem services. Ecological Monographs 81: 169–193. https://doi.org/10.1890/10-1510.1.
Barbour, M.A., M.A. Rodriguez-Cabal, E.T. Wu, R. Julkunen-Tiitto, C.E. Ritland, A.E. Miscampbell, E.S. Jules, and G.M. Crutsinger. 2015. Multiple plant traits shape the genetic basis of herbivore community assembly. Functional Ecology 29: 995–1006. https://doi.org/10.1111/1365-2435.12409.
Bayraktarov, E., M.I. Saunders, S. Abdullah, M. Mills, J. Beher, H.P. Possingham, P.J. Mumby, and C.E. Lovelock. 2016. The cost and feasibility of marine coastal restoration. Ecological Applications 26. Wiley Online Library: 1055–1074.
Bernhardt, J.R., and H.M. Leslie. 2013. Resilience to climate change in coastal marine ecosystems. Annual Review of Marine Science 5: 371–392. https://doi.org/10.1146/annurev-marine-121211-172411.
Bertness, M.D. 1984. Ribbed mussels and Spartina alterniflora production in a New England salt marsh. Ecology 65: 1794–1807. https://doi.org/10.2307/1937776.
Bilkovic, D.M., R.E. Isdell, A.G. Guthrie, M.M. Mitchell, and R.M. Chambers. 2021. Ribbed mussel Geukensia demissa population response to living shoreline design and ecosystem development. Ecosphere 12: e03402. https://doi.org/10.1002/ecs2.3402.
Bischoff, A., T. Steinger, and H. Müller-Schärer. 2010. The importance of plant provenance and genotypic diversity of seed material used for ecological restoration. Restoration Ecology 18. John Wiley & Sons, Ltd (10.1111): 338–348. https://doi.org/10.1111/j.1526-100X.2008.00454.x.
Blum, M J., K. Jun Bando, M. Katz, and D.R. Strong. 2007. Geographic structure, genetic diversity and source tracking of Spartina alterniflora. Journal of Biogeography 34. John Wiley & Sons, Ltd (10.1111): 2055–2069. https://doi.org/10.1111/j.1365-2699.2007.01764.x.
Blum, M.J., C.M. Sloop, D.R. Ayres, and D.R. Strong. 2004. Characterization of microsatellite loci in Spartina species (Poaceae). Molecular Ecology Notes 4. John Wiley & Sons, Ltd (10.1111): 39–42. https://doi.org/10.1046/j.1471-8286.2003.00556.x.
Breder, C.M. 1960. Design for a fry trap. Zoologica 45: 155–160.
Bromberg, K.D., and M.D. Bertness. 2005. Reconstructing New England salt marsh losses using historical maps. Estuaries 28: 823–832. https://doi.org/10.1007/BF02696012.
Cahoon, D.R., K.L. McKee, and J.T. Morris. 2021. How plants influence resilience of salt marsh and mangrove wetlands to sea-level rise. Estuaries and Coasts 44: 883–898. https://doi.org/10.1007/s12237-020-00834-w.
Campbell, A.D., L. Fatoyinbo, L. Goldberg, and D. Lagomasino. 2022. Global hotspots of salt marsh change and carbon emissions. Nature 612. Nature Publishing Group: 701–706. https://doi.org/10.1038/s41586-022-05355-z.
Coleman, D.J., M. Schuerch, S. Temmerman, G. Guntenspergen, C.G. Smith, and M.L. Kirwan. 2022. Reconciling models and measurements of marsh vulnerability to sea level rise. Limnology and Oceanography Letters 7: 140–149. https://doi.org/10.1002/lol2.10230.
Craft, C., J. Reader, J.N. Sacco, and S.W. Broome. 1999. Twenty-five years of ecosystem development of constructed Spartina Alterniflora (loisel) marshes. Ecological Applications 9: 1405–1419. https://doi.org/10.1890/1051-0761(1999)009[1405:TFYOED]2.0.CO;2.
Craft, C., and J. Sacco. 2003. Long-term succession of benthic infauna communities on constructed Spartina alterniflora marshes. Marine Ecology Progress Series 257: 45–58. https://doi.org/10.3354/meps257045.
Crosby, S.C., A. Angermeyer, J.M. Adler, M.D. Bertness, L.A. Deegan, N. Sibinga, and H.M. Leslie. 2017. Spartina alterniflora biomass allocation and temperature: Implications for salt marsh persistence with sea-level rise. Estuaries and Coasts 40: 213–223. https://doi.org/10.1007/s12237-016-0142-9.
Crosby, S.C., D.F. Sax, M.E. Palmer, H.S. Booth, L.A. Deegan, M.D. Bertness, and H.M. Leslie. 2016. Salt marsh persistence is threatened by predicted sea-level rise. Estuarine, Coastal and Shelf Science. https://doi.org/10.1016/j.ecss.2016.08.018.
Crotty, S.M., D. Pinton, A. Canestrelli, H.S. Fischman, C. Ortals, N.R. Dahl, S. Williams, T.J. Bouma, and C. Angelini. 2023. Faunal engineering stimulates landscape-scale accretion in southeastern US salt marshes. Nature Communications 14. Nature Publishing Group: 881. https://doi.org/10.1038/s41467-023-36444-w.
Crutsinger, G.M., M.D. Collins, J.A. Fordyce, Z. Gompert, C.C. Nice, and N.J. Sanders. 2006. Plant genotypic diversity predicts community structure and governs an ecosystem process. Science 313. American Association for the Advancement of Science: 966–968. https://doi.org/10.1126/science.1128326.
Deegan, L.A., D.S. Johnson, R.S. Warren, B.J. Peterson, J.W. Fleeger, S. Fagherazzi, and W.M. Wollheim. 2012. Coastal eutrophication as a driver of salt marsh loss. Nature 490. Nature Publishing Group, a division of Macmillan Publishers Limited. All Rights Reserved.: 388.
Derksen-Hooijberg, M., T. van der Heide, L.P.M. Lamers, A. Borst, A.J.P. Smolders, L.L. Govers, J.R.H. Hoogveld, and C. Angelini. 2019. Burrowing crabs weaken mutualism between foundation species. Ecosystems 22: 767–780. https://doi.org/10.1007/s10021-018-0301-x.
Ehlers, A., B. Worm, and T.B.H. Reusch. 2008. Importance of genetic diversity in eelgrass Zostera marina for its resilience to global warming. Marine Ecology Progress Series 355: 1–7. https://doi.org/10.3354/meps07369.
Evans, L.M., J.S. Clark, A.V. Whipple, and T.G. Whitham. 2012. The relative influences of host plant genotype and yearly abiotic variability in determining herbivore abundance. Oecologia 168: 483–489. https://doi.org/10.1007/s00442-011-2108-8.
Fagherazzi, S., G. Mariotti, N. Leonardi, A. Canestrelli, W. Nardin, and W.S. Kearney. 2020. Salt marsh dynamics in a period of accelerated sea level rise. Journal of Geophysical Research: Earth Surface 125: e2019JF005200. https://doi.org/10.1029/2019JF005200.
FitzGerald, D.M., and Z. Hughes. 2019. Marsh processes and their response to climate change and sea-level rise. Annual Review of Earth and Planetary Sciences 47: 481–517. https://doi.org/10.1146/annurev-earth-082517-010255.
Foust, C.M., V. Preite, A.W. Schrey, M. Alvarez, M.H. Robertson, K.J.F. Verhoeven, and C.L. Richards. 2016. Genetic and epigenetic differences associated with environmental gradients in replicate populations of two salt marsh perennials. Molecular Ecology 25: 1639–1652. https://doi.org/10.1111/mec.13522.
Gallagher, J.L., G.F. Somers, D.M. Grant, and D.M. Seliskar. 1988. Persistent differences in two forms of Spartina alterniflora: a common garden experiment. Ecology 69. John Wiley & Sons, Ltd: 1005–1008. https://doi.org/10.2307/1941255.
Gaynor, M.L., L.J. Walters, and E.A. Hoffman. 2019. Ensuring effective restoration efforts with salt marsh grass populations by assessing genetic diversity. Restoration Ecology 27: 1452–1462. https://doi.org/10.1111/rec.13014.
Grabowski, J.H., R.D. Brumbaugh, R.F. Conrad, A.G. Keeler, J.J. Opaluch, C.H. Peterson, M.F. Piehler, S.P. Powers, and A.R. Smyth. 2012. Economic valuation of ecosystem services provided by oyster reefs. BioScience 62: 900–909. https://doi.org/10.1525/bio.2012.62.10.10.
Hughes, A.R. 2014. Genotypic diversity and trait variance interact to affect marsh plant performance. Journal of Ecology 102. Wiley Online Library: 651–658.
Hughes, A.R., B.D. Inouye, M.T.J. Johnson, N. Underwood, and M. Vellend. 2008. Ecological consequences of genetic diversity. Ecology Letters 11. John Wiley & Sons, Ltd (10.1111): 609–623. https://doi.org/10.1111/j.1461-0248.2008.01179.x.
Hughes, A.R., and K.E. Lotterhos. 2014. Genotypic diversity at multiple spatial scales in the foundation marsh species, Spartina alterniflora. Marine Ecology Progress Series 497: 105–117.
Hughes, A.R., A.F.P. Moore, and M.F. Piehler. 2014. Independent and interactive effects of two facilitators on their habitat-providing host plant, Spartina alterniflora. Oikos 123. John Wiley & Sons, Ltd (10.1111): 488–499. https://doi.org/10.1111/j.1600-0706.2013.01035.x.
Johnson, M.T.J., M. Vellend, and J.R. Stinchcombe. 2009. Evolution in plant populations as a driver of ecological changes in arthropod communities. Philosophical Transactions of the Royal Society b: Biological Sciences 364: 1593–1605. https://doi.org/10.1098/rstb.2008.0334.
Jones, T.A. 2003. The restoration gene pool concept: beyond the native versus non-native debate. Restoration Ecology 11. John Wiley & Sons, Ltd (10.1111): 281–290. https://doi.org/10.1046/j.1526-100X.2003.00064.x.
Jones, T.A. 2013. When local isn’t best. Evolutionary Applications 6. John Wiley & Sons, Ltd (10.1111): 1109–1118. https://doi.org/10.1111/eva.12090.
Kettenring, K.M., K.L. Mercer, C. Reinhardt Adams, and J. Hines. 2014. Application of genetic diversity–ecosystem function research to ecological restoration. Journal of Applied Ecology 51. John Wiley & Sons, Ltd (10.1111): 339–348. https://doi.org/10.1111/1365-2664.12202.
Kirwan, M.L., G. Guntenspergen, and J.T. Morris. 2009. Latitudinal trends in Spartina alterniflora productivity and the response of coastal marshes to global change. Global Change Biology 15. John Wiley & Sons, Ltd (10.1111): 1982–1989. https://doi.org/10.1111/j.1365-2486.2008.01834.x.
Lawrence, P.J., G.R. Smith, M.J.P. Sullivan, and H.L. Mossman. 2018. Restored saltmarshes lack the topographic diversity found in natural habitat. Ecological Engineering 115: 58–66. https://doi.org/10.1016/j.ecoleng.2018.02.007.
Liu, Z., S. Fagherazzi, Q. He, O. Gourgue, J. Bai, X. Liu, C. Miao, Z. Hu, and B. Cui. 2024. A global meta-analysis on the drivers of salt marsh planting success and implications for ecosystem services. Nature Communications 15. Nature Publishing Group: 3643. https://doi.org/10.1038/s41467-024-47769-5.
McKay, J.K., C.E. Christian, S. Harrison, and K.J. Rice. 2005. “How Local Is Local?”—a review of practical and conceptual issues in the genetics of restoration. Restoration Ecology 13. John Wiley & Sons, Ltd (10.1111): 432–440. https://doi.org/10.1111/j.1526-100X.2005.00058.x.
Meirmans, P.G. 2020. GENODIVE version 3.0: Easy-to-use software for the analysis of genetic data of diploids and polyploids. Molecular Ecology Resources 20: 1126–1131. https://doi.org/10.1111/1755-0998.13145.
Mossman, H.L., A.J. Davy, and A. Grant. 2012. Does managed coastal realignment create saltmarshes with ‘equivalent biological characteristics’ to natural reference sites? Journal of Applied Ecology 49: 1446–1456. https://doi.org/10.1111/j.1365-2664.2012.02198.x.
Nef, D.P., E. Gotor, G. Wiederkehr Guerra, M. Zumwald, and C.J. Kettle. 2021. Initial investment in diversity is the efficient thing to do for resilient forest landscape restoration. Frontiers in Forests and Global Change 3.
Noto, A.E., and A.R. Hughes. 2020. Intraspecific diversity at two trophic levels influences plant–herbivore interactions. Ecosphere 11: e03121. https://doi.org/10.1002/ecs2.3121.
Novy, A., J.M. Hartman, L. Struwe, P. Smouse, J. Honig, C. Miller, and S. Bonos. 2008. Patterns of genetic variation of Spartina alterniflora in marshes of the New York metropolitan area and significance for marsh restoration.
Novy, A., P.E. Smouse, J.M. Hartman, L. Struwe, J. Honig, C. Miller, M. Alvarez, and S. Bonos. 2010. Genetic variation of Spartina alterniflora in the New York metropolitan area and its relevance for marsh restoration. Wetlands 30: 603–608. https://doi.org/10.1007/s13157-010-0046-6.
Plaisted, H.K., A.B. Novak, S. Weigel, A.S. Klein, and F.T. Short. 2020. Eelgrass genetic diversity influences resilience to stresses associated with eutrophication. Estuaries and Coasts 43: 1425–1438. https://doi.org/10.1007/s12237-019-00669-0.
R Core Team. 2020. R: a language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing.
Reynolds, L.K., K.J. McGlathery, and M. Waycott. 2012. Genetic diversity enhances restoration success by augmenting ecosystem services. PLOS ONE 7. Public Library of Science: e38397. https://doi.org/10.1371/journal.pone.0038397.
Rezek, R.J., B. Lebreton, B. Sterba-Boatwright, and J.B. Pollack. 2017. Ecological structure and function in a restored versus natural salt marsh. PLOS ONE 12. Public Library of Science: e0189871. https://doi.org/10.1371/journal.pone.0189871.
Richards, C.L., J.L. Hamrick, L.A. Donovan, and R. Mauricio. 2004. Unexpectedly high clonal diversity of two salt marsh perennials across a severe environmental gradient. Ecology Letters 7: 1155–1162. https://doi.org/10.1111/j.1461-0248.2004.00674.x.
Robertson, M., A. Schrey, A. Shayter, C.J. Moss, and C. Richards. 2017. Genetic and epigenetic variation in Spartina alterniflora following the Deepwater Horizon oil spill. Evolutionary Applications 10: 792–801. https://doi.org/10.1111/eva.12482.
RStudio Team (2020). RStudio: integrated development for R. RStudio, PBC, Boston, MA URL http://www.rstudio.com/. Boston, MA: RStudio.
Schaberg, P.G., D.H. DeHayes, G.J. Hawley, and S.E. Nijensohn. 2008. Anthropogenic alterations of genetic diversity within tree populations: Implications for forest ecosystem resilience. Forest Ecology and Management 256: 855–862. https://doi.org/10.1016/j.foreco.2008.06.038.
Schuerch, M., T. Spencer, S. Temmerman, M.L. Kirwan, C. Wolff, D. Lincke, C.J. McOwen, et al. 2018. Future response of global coastal wetlands to sea-level rise. Nature 561. Nature Publishing Group: 231–234. https://doi.org/10.1038/s41586-018-0476-5.
Shea, M.L., R.S. Warren, and W.A. Niering. 1975. Biochemical and transplantation studies of the growth form of Spartina alterniflora on Connecticut salt marshes. Ecology 56. John Wiley & Sons, Ltd: 461–466. https://doi.org/10.2307/1934977.
Sloop, C.M., H.G. McGray, M.J. Blum, and D.R. Strong. 2005. Characterization of 24 additional microsatellite loci in Spartina species (Poaceae). Conservation Genetics 6: 1049–1052. https://doi.org/10.1007/s10592-005-9084-7.
Smith, S.M., and S. Warren. 2007. Determining ground surface topography in tidal marshes using watermarks. Journal of Coastal Research 23: 265–269. https://doi.org/10.2112/05-0522R.1.
Temmink, R.J.M., C. Angelini, M. Verkuijl, and T. van der Heide. 2023. Restoration ecology meets design-engineering: Mimicking emergent traits to restore feedback-driven ecosystems. Science of the Total Environment 902: 166460. https://doi.org/10.1016/j.scitotenv.2023.166460.
Thompson, S.P., H.W. Paerl, and M.C. Go. 1995. Seasonal patterns of nitrification and denitrification in a natural and a restored salt marsh. Estuaries 18: 399–408. https://doi.org/10.2307/1352322.
Timpane-Padgham, B.L., T. Beechie, and T. Klinger. 2017. A systematic review of ecological attributes that confer resilience to climate change in environmental restoration. PLOS ONE 12. Public Library of Science: e0173812. https://doi.org/10.1371/journal.pone.0173812.
Travis, S.E., and J.B. Grace. 2010. Predicting performance for ecological restoration: a case study using Spartina alterniflora. Ecological Applications 20. John Wiley & Sons, Ltd: 192–204. https://doi.org/10.1890/08-1443.1.
Travis, S.E., C.E. Proffitt, R.C. Lowenfeld, and T.W. Mitchell. 2002. A comparative assessment of genetic diversity among differently-aged populations of Spartina alterniflora on restored versus natural wetlands. Restoration Ecology 10. John Wiley & Sons, Ltd (10.1111): 37–42. https://doi.org/10.1046/j.1526-100X.2002.10104.x.
Vahsen, M.L., M.J. Blum, J.P. Megonigal, S.J. Emrich, J.R. Holmquist, B. Stiller, K. E.O. Todd-Brown, and J.S. McLachlan. 2023. Rapid plant trait evolution can alter coastal wetland resilience to sea level rise. Science 379. American Association for the Advancement of Science: 393–398. https://doi.org/10.1126/science.abq0595.
Valiela, I., J.M. Teal, and W.G. Deuser. 1978. The nature of growth forms in the salt marsh grass Spartina alterniflora. The American Naturalist 112. The University of Chicago Press: 461–470. https://doi.org/10.1086/283290.
Walker, J.B., A.L. Bijak, and L. Blum. 2021. Genetic diversity and clonal structure of Spartina alterniflora in a Virginia marsh. Northeastern Naturalist 28. Eagle Hill Institute: 357–370. https://doi.org/10.1656/045.028.0309.
Wang, F., M. Eagle, K.D. Kroeger, A.C. Spivak, and J. Tang. 2021. Plant biomass and rates of carbon dioxide uptake are enhanced by successful restoration of tidal connectivity in salt marshes. Science of the Total Environment 750: 141566. https://doi.org/10.1016/j.scitotenv.2020.141566.
Warren, R.S., P.E. Fell, R. Rozsa, A.H. Brawley, A.C. Orsted, E.T. Olson, V. Swamy, and W.A. Niering. 2002. Salt marsh restoration in Connecticut: 20 years of science and management. Restoration Ecology 10. John Wiley & Sons, Ltd (10.1111): 497–513. https://doi.org/10.1046/j.1526-100X.2002.01031.x.
Watson, E.B., A.J. Oczkowski, C. Wigand, A.R. Hanson, E.W. Davey, S.C. Crosby, R.L. Johnson, and H.M. Andrews. 2014. Nutrient enrichment and precipitation changes do not enhance resiliency of salt marshes to sea level rise in the Northeastern U.S. Climatic Change 125: 501–509. https://doi.org/10.1007/s10584-014-1189-x.
Wei, X., Y. Xu, L. Lyu, Z. Xiao, S. Wang, T. Yang, and M. Jiang. 2023. Impacts of ecological restoration on the genetic diversity of plant species: A global meta-analysis. Journal of Applied Ecology 60: 1149–1160. https://doi.org/10.1111/1365-2664.14390.
Weston, N.B. 2014. Declining sediments and rising seas: An unfortunate convergence for tidal wetlands. Estuaries and Coasts 37: 1–23. https://doi.org/10.1007/s12237-013-9654-8.
Whaley, T., and M. Alldred. 2023. A meta-analysis reveals knowledge gaps in our understanding of the Spartina-Geukensia mutualism. Estuaries and Coasts 46: 1021–1034. https://doi.org/10.1007/s12237-023-01194-x.
Whitham, T.G., W.P. Young, G.D. Martinsen, C.A. Gehring, J.A. Schweitzer, S.M. Shuster, G.M. Wimp, et al. 2003. Community and ecosystem genetics: A consequence of the extended phenotype. Ecology 84: 559–573. https://doi.org/10.1890/0012-9658(2003)084[0559:CAEGAC]2.0.CO;2.
Williams, S.L. 2001. Reduced genetic diversity in eelgrass transplantations affects both population growth and individual fitness. Ecological Applications 11: 1472–1488. https://doi.org/10.1890/1051-0761(2001)011[1472:RGDIET]2.0.CO;2.
Wimp, G.M., W.P. Young, S.A. Woolbright, G.D. Martinsen, P. Keim, and T.G. Whitham. 2004. Conserving plant genetic diversity for dependent animal communities. Ecology Letters 7: 776–780. https://doi.org/10.1111/j.1461-0248.2004.00635.x.
Zerebecki, R.A., G.M. Crutsinger, and A.R. Hughes. 2017. Spartina alterniflora genotypic identity affects plant and consumer responses in an experimental marsh community. Journal of Ecology 105: 661–673. https://doi.org/10.1111/1365-2745.12703.
Zerebecki, R.A., and A.R. Hughes. 2023. Ecological consequences of genotypic diversity within a foundation plant, Spartina alterniflora, are pervasive but not universal across multiple stress gradients. Journal of Ecology 111. Wiley-Blackwell. https://doi.org/10.1111/1365-2745.14147.
Zerebecki, R.A., E.E. Sotka, T.C. Hanley, K.L. Bell, C. Gehring, C.C. Nice, C.L. Richards, and A.R. Hughes. 2021. Repeated genetic and adaptive phenotypic divergence across tidal elevation in a foundation plant species. The American Naturalist 198. The University of Chicago Press: E152–E169. https://doi.org/10.1086/716512.
Zogg, G.P., and S.E. Travis. 2022. Genotypic diversity reduces the negative effects of increased competition, herbivory, and tidal inundation on the productivity of Spartina alterniflora. Estuaries and Coasts 45: 462–469. https://doi.org/10.1007/s12237-021-00962-x.
Acknowledgements
The authors wish to extend their sincere thanks to the late Dr. Jennifer Mattei, whose study site access, knowledge, kindness, humor, and partnership greatly benefited the project. The authors wish to thank The Connecticut Department of Transportation (especially Christopher Samorajczyk), Branford Land Trust, Milford Land Conservation Trust, East Haven Land Trust, Groton-New London Airport (especially Ethan Temple and Shawn McComiskey), the Groton Long Point Association, The Nature Conservancy, and Bridgeport-Sikorsky Airport for their assistance with study site access and/or restoration site information. Sarah Sherts, Grace Bucci, Julia Frees, Cosmo Randazzo, Lars Vikstrom, Kristen Lodato, Al Nyack, Tyler Mahard, Adrian Nelson, Zach Weier, Sophia Ruh, Nick Balfour, and Thomas Hoffmeister provided invaluable assistance with field data collection. Heather Heenehan and Greens Farms Academy are thanked for their assistance with access to the salt marsh site used in the common garden study. Shimon Anisfeld generously provided use of a real-time kinematics unit for the elevation measurements in this study. We appreciate the support and guidance of Juliana Barrett, Syma Ebbin, Sylvain De Guise, and Judy Preston, especially as the research timeline was forced to shift due to the pandemic.
Funding
This work was provided by Connecticut Sea Grant, University of Connecticut, through Award Number NA18OAR4170081, Project Number 390251. These data and related items of information have not been formally disseminated by NOAA and do not represent any agency determination, view, or policy.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design, and data collection and analysis. The first draft of the manuscript was written by Sarah Crosby and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics Approval
The authors have no relevant ethical considerations to disclose.
Consent to Participate
Not applicable.
Competing Interests
The authors declare no competing interests.
Additional information
Communicated by John Carroll
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Crosby, S.C., Hudson, D.M., Hughes, A.R. et al. Structure and Function of Restored and Natural Salt Marshes: Implications for Ecosystem Resilience and Adaptive Potential. Estuaries and Coasts 47, 1561–1578 (2024). https://doi.org/10.1007/s12237-024-01395-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12237-024-01395-y