Abstract
The Ria Formosa coastal lagoon is a highly productive shallow ecosystem in southern Portugal, subjected to nutrient inputs from anthropogenic and natural sources. Nutrients are major abiotic drivers of phytoplankton in this system, but their effects on phytoplankton assemblages and the occurrence of nutrient limitation are still poorly understood. The main goal of this study was, thus, to evaluate the occurrence, type, and effects of nutrient limitation on phytoplankton community and specific functional groups in the Ria Formosa coastal lagoon. We conducted nutrient enrichment experiments with factorial additions of nitrogen (N) and phosphorus (P) using natural phytoplankton assemblages from distinct locations in the Ria Formosa, throughout a yearly cycle. Phytoplankton composition and abundance were evaluated using inverted and epifluorescence microscopies, and spectrophotometric methods were used for biomass. Limitation was defined as higher phytoplankton growth following enrichment with a particular nutrient in relation to the non-enriched control. The most common type of phytoplankton limitation was simultaneous co-limitation by N and P; diatoms, as r-strategists, were the most frequently limited group. Single N and P limitation, and serial P limitation were also observed, as well as negative responses to nutrient enrichment. Group-specific responses to nutrient enrichment were not reflected in the relative abundance of phytoplankton groups within the whole assemblage, due to the numerical dominance of pico-sized groups (cyanobacteria and eukaryotic picophytoplankton). Ambient nutrient ratios and concentrations did not predict phytoplankton nutrient limitation, given the different nutrient utilisation traits among phytoplankton functional groups. Therefore, nutrient ratios should not be used as indicators of nutrient limitation in eutrophication assessment.
Similar content being viewed by others
Introduction
Coastal lagoons are complex socio-ecological systems that rank among the most biologically productive and important ecosystems on the Planet, providing goods and services valuable for human welfare (Kennish and Paerl 2010; Newton et al. 2018). However, these systems are also increasingly exposed to anthropogenic pressures associated with population growth and land-use alterations, which may hinder their ability to provide ecosystem services. One of the main consequences of anthropogenic activities in coastal lagoons is nutrient over-enrichment; loads of nitrogen and phosphorus have been increasing due to fertiliser use, animal waste, industrial pollution, or atmospheric deposition, causing nutrient pollution that ultimately results in eutrophication (Glibert et al. 2018). Nutrient inputs of natural origins, such as coastal upwelling events, can also promote natural eutrophication episodes in coastal lagoons, as these ecosystems are located at the interface between land and the ocean.
Nutrient over-enrichment has been addressed for decades, but eutrophication remains a leading challenge in coastal ecosystems. Eutrophication has been associated with the development of harmful algal blooms (HABs) (Heisler et al. 2008; Glibert and Burford 2017), thus contributing to deleterious impacts on ecosystem functioning, public health, tourism, and fisheries (Anderson et al. 2012). In addition, climate change may intensify the symptoms of eutrophication (Jeppesen et al. 2010), especially in shallow confined coastal ecosystems, adding to the challenge of restoring water quality and ecosystem health. Therefore, information on how excessive nutrients affect phytoplankton growth and community composition is essential for coastal management.
Different strategies can be used to assess phytoplankton nutrient limitation, namely the determination of nutrient uptake kinetics for specific phytoplankton taxa or the establishment of nutrient criteria, based on nutrient concentrations and ratios (Ren et al. 2009). Other strategies, such as ambient elemental ratios, macromolecular composition of cells, and molecular and biochemical approaches, can also be employed (Beardall et al. 2001). The most frequently used approach is nutrient addition bioassays (Beardall et al. 2001; Ren et al. 2009; Domingues et al. 2017a), whereby specific nutrients are added, alone and/or in combinations, to natural phytoplankton assemblages. Potential nutrient limitation is identified by higher phytoplankton growth following enrichment with a particular nutrient in relation to the control (Domingues et al. 2011). The occurrence of nutrient limitation and the relative importance of each nutrient is not straightforward, as nutrient limitation in phytoplankton may not follow Liebig’s Law of the Minimum, i.e. of single-nutrient limitation. Instead, interactions such as co-limitation by several nutrients (or other resources) are widespread across aquatic ecosystems (Elser et al. 2007), and vary on different spatial and temporal scales, rendering it difficult to classify an ecosystem as N- or P-limited and to establish specific nutrient limitation criteria. Given the importance of nutrient concentrations and ratios for the evaluation of eutrophication, understanding the occurrence and variability of nutrient limitation in coastal lagoons is essential for a successful management of eutrophication in these ecosystems.
The Ria Formosa is a shallow coastal lagoon system (southern Portugal) subjected to anthropogenic and natural nutrient enrichments and located in an area extremely vulnerable to climate change (Arias et al. 2021). The Ria Formosa is one of the most important ecosystems in Portugal, from biological and socio-economic perspectives, since it serves as a breeding and feeding ground for many species of fish and birds, and supports different human activities, including fishing, shellfish farming, aquaculture, and tourism (Barbosa 2010; Newton et al. 2020). The lagoon is subjected to several anthropogenic sources of nutrients, such as discharges of treated domestic and industrial sewage, and runoffs from golf courses and agriculture, which have been associated with increasing nutrient concentrations in the lagoon (Newton et al. 2014; Cravo et al. 2015, 2022; Jacob et al. 2020). HABs and non-harmful phytoplankton blooms have been reported in tandem with other eutrophication symptoms, such as water deoxygenation, anoxic sediments, opportunistic green macroalgae blooms, decreased benthos and fish biodiversity, and fish kills (Newton et al. 2014). These indicators have been associated with large inputs of N and P, and unbalanced N:P and Si:N ratios (Newton et al. 2014). However, a generalised improvement in water quality has been detected in recent years in the Ria Formosa in relation to the early 2000’s, due to the upgrade of wastewater treatment systems (Cravo et al. 2018; Rosa et al. 2022). Eutrophication symptoms in the Ria Formosa are only observed in the vicinity of wastewater and industrial discharges, as the high semidiurnal tidal prisms and low water residence time promote flushing of nutrients and contaminants to adjacent waters (Cravo et al. 2014, 2018, 2022), thus contributing to the lagoon’s robustness and reduced susceptibility to eutrophication (Barbosa 2010; Cravo et al. 2022).
In shallow ecosystems like the Ria Formosa, where light limitation seldom occurs, nutrients are a major abiotic driver of phytoplankton (Domingues et al. 2017a). However, the occurrence and type of nutrient limitation and the role of nutrients on phytoplankton growth and community composition in the Ria Formosa coastal lagoon are still poorly understood. The effects of nutrient enrichments on phytoplankton were firstly investigated in two lagoon locations, but only during late spring and early summer periods (Loureiro et al. 2005). Later studies have analysed the effects of nutrient and light enrichments on phytoplankton in shallow, mostly inner lagoon locations (Domingues et al. 2015, 2017a, b). The inner lagoon domains, representative of the land and urban boundaries of the Ria Formosa, are characterised by higher nutrient and light availability, and a weaker hydrodynamic regime (Cravo et al. 2022). In contrast, outer, deeper, less studied lagoon areas, such as main navigational channels and inlets, are subjected to a strong hydrodynamic regime and more exposed to the influence of the adjacent coastal waters, being impacted by regular upwelling events (Relvas et al. 2007). These outer lagoon areas are also more susceptible to the advection of HABs occurring in adjacent coastal waters (Cravo et al. 2022; Lima et al. 2022).
The main goal of this study was to evaluate the occurrence, type, and effects of nutrient limitation on phytoplankton in the Ria Formosa coastal lagoon, covering both inner and outer lagoon domains and all seasons of the year. Specific questions to be answered were as follows: (1) What are the effects of nutrient enrichment on the abundance of specific phytoplankton functional groups and composition of phytoplankton assemblages? and (2) Can nutrient stoichiometry be used to evaluate nutrient limitation and phytoplankton responses to nutrient enrichment? To address these research questions, we conducted nutrient enrichment microcosm experiments using natural phytoplankton from the Ria Formosa. Based on previous experimental (Domingues et al. 2015, 2017a, b) and observational studies (Cravo et al. 2022) conducted in the Ria Formosa, we hypothesise that nitrogen is the single limiting nutrient for phytoplankton, particularly during the productive periods. We also anticipate a stronger nitrogen limitation in the outer lagoon intensified for r-strategist phytoplankton groups such as diatoms.
Materials and Methods
Study Site
The Ria Formosa coastal lagoon (Fig. 1) is located in southern Portugal (SW Europe), in a highly vulnerable area to climate change (Arias et al. 2021). The lagoon is an euryhaline, shallow (mean depth = 2 m; Barbosa 2010; Cravo et al. 2014), and mesotidal system with semidiurnal tides, separated from the Atlantic Ocean by a multi-inlet sandy barrier island system that extends approximately 55 km E-W and 6 km N-S at its widest point. The region is subjected to hot dry summers and moderate winters, typical of Mediterranean climate. Lagoon dynamics is strongly influenced by oceanographic and meteorological processes in the adjacent coastal zone, particularly regarding its chemical characteristics (Cravo et al. 2019). The outer section of the lagoon is impacted by regular upwelling events (Loureiro et al. 2006; Barbosa 2010), that may extend approx. 6 km upstream from the lagoon inlets (Cravo et al. 2014). The Ria Formosa system, with a wet surface area of 105 km2 and a total area of 185 km2, is protected by multiple national and international policies (see Barbosa 2010), due to its ecological and socio-economic importance.
This study was carried out at two distinct locations in the western sector of the Ria Formosa coastal lagoon (Fig. 1): the inner lagoon, representative of the landward boundary, and the outer lagoon (Faro-Olhão inlet), representative of the seaward boundary. A full description of the environmental characteristics of these locations during the sampling period (2011–2012) can be found elsewhere (Domingues et al. 2015).
N and P Enrichment Experiments
Four experiments were conducted during representative seasons for phytoplankton growth, throughout 2011–2012: autumn (early December 2011), winter (February 2012), spring (late March 2012), and summer (June–July 2012). Sub-surface water samples were collected at the inner and outer lagoon sampling stations during flood tide into transparent 2 L polycarbonate bottles (Nalgene). Samples were not pre-screened to eliminate larger grazers, given that this procedure would also remove larger, colonial phytoplankton species, leading to significant alterations in the structure of the initial phytoplankton assemblage, thus increasing the problems associated with the extrapolation of experimental results to the natural ecosystem (Nogueira et al. 2014). Four experimental treatments (control, N, P, NP) were prepared and two replicates were used, due to the amount of work that the experimental procedure entails, particularly the time-consuming microscopy analysis. Nutrients were added in a single, saturating pulse, based on previous reports of nutrient concentrations in the Ria Formosa lagoon (see review by Barbosa 2010). Nutrient concentrations added were the following: C – no additions; N – 100 µM potassium nitrate; P – 10 µM potassium dihydrogen phosphate; NP – 100 µM nitrate + 10 µM phosphate). Silicon was not added given that previous nutrient enrichment experiments reported that silicon was never limiting to phytoplankton growth (Domingues et al. 2015, 2017a). The bottles were incubated in situ for 24 h (summer experiments) or 48 h (autumn, winter, and spring experiments), fixed to a mooring buoy, exposed to ambient temperature, ambient light, and natural light–dark cycle, and were continuously shaken by tidal currents and wind. Ambient light exposure was obtained by covering the bottles with different layers of net, to simulate the mean light intensity in the mixed layer. Samples for determination of dissolved inorganic macronutrient concentrations, chlorophyll-a concentration, and phytoplankton composition and abundance were collected from each bottle at the beginning and end of the incubation period.
Environmental Variables
Vertical profiles of photosynthetically active radiation (PAR), measured with a LI-COR LI-193 spherical underwater quantum sensor, were used to calculate the light extinction coefficient (Ke) as Iz = I0 e-Ke.Z, where Iz is the light intensity at depth level Z (m) and I0 is the light intensity at the surface. The mean light intensity in the mixed layer (Im) was then calculated as Io (1-e(−Ke.Zm))(Ke.Zm)−1 where Zm is the depth of the mixed layer (m), which, in the Ria Formosa, corresponds to the whole water column, due to the absence of haline and thermal stratification.
Spectrophotometric methods described by Grasshoff et al. (1999) were used to determine concentrations of dissolved inorganic macronutrients. Water samples were collected, filtered through cellulose acetate filters (Whatman, nominal pore diameter = 0.2 µm), and frozen until analysis. Ammonium (NH4+), phosphate (PO43−), and silicate (SiO44−) were determined within 24 h of sample collection using the spectrophotometer Hitachi U-2000, whilst samples for nitrate (NO3−) and nitrite (NO2−) where frozen (− 20 °C) until analysis on an Skalar SAN + continuous flow analyser. The concentration of dissolved inorganic nitrogen (DIN) was calculated as the sum of NO3−, NO2−, and NH4+.
Water samples for determination of chlorophyll-a concentration were filtered through glass fibre filters (Whatman GF/F, retention > 0.7 µm) and pigments were extracted overnight at 4 °C with 90% acetone. After centrifugation, absorbance of the supernatant was measured spectrophotometrically (Hitachi U-2000) at 750 and 665 nm, before and after acidification with HCl 1 M (Parsons et al. 1984).
Composition and abundance of pico- (< 2 µm) and nanophytoplankton (2–20 µm) were determined using epifluorescence microscopy (Haas 1982), whilst inverted microscopy (Utermöhl 1958) was used for microphytoplankton (> 20 µm). Water samples for epifluorescence microscopy were preserved immediately after collection with glutardialdehyde (final concentration 2%), stained with proflavine, and filtered onto black polycarbonate membrane filters (Whatman, nominal pore diameter = 0.45 µm), within 24 h of sampling. Preparations were made using glass slides and non-fluorescent immersion oil (Cargille type A), and frozen (− 20 °C) in dark conditions, to minimise loss of autofluorescence. Cell enumeration was made at × 1000 magnification using a Zeiss Axio Imager A1 epifluorescence microscope. Samples for inverted microscopy were preserved immediately after collection with acid Lugol’s solution (final concentration approx. 0.003%), settled in sedimentation chambers, and observed at × 400 magnification with a Zeiss Axio Observer A1 inverted microscope. A minimum of 50 random visual fields, at least 400 cells in total, and 50 cells of the most common genus were counted, for a counting precision of ± 10%, assuming the cells were randomly distributed (Venrick 1978). Phytoplankton was identified into major functional groups, namely: diatoms, dinoflagellates, cryptophytes, other autotrophic nanoflagellates, eukaryotic picophytoplankton, and cyanobacteria.
All material used in the experiments and laboratorial analyses was previously chemically decontaminated with HCl 10% and NaOH 1 g L−1, and thoroughly rinsed with deionised water.
Evaluation of Nutrient Limitation
The occurrence of potential nutrient limitation was based on ambient (observational) nutrient data and experimental data. Potential nutrient limitation at the beginning of the experiments was evaluated according to the resource-ratio hypothesis, that predicts that the outcome of interspecific competition depends on the nutrient supply ratios (Tilman 1985). To evaluate if ambient NP ratios accurately predict the type of nutrient limitation, ambient nutrient concentrations were plotted on a biplot with delineated regions of hypothesised N and P limitation (see Burson et al. 2018; Lewis et al. 2020). The nutrient molar ratios used as thresholds were those proposed by (Guildford and Hecky 2000): N limitation for N:P < 20 and P limitation for N:P > 50. Nutrient limitation criteria that use both nutrient ratios and absolute concentrations were also used, according to: N limitation if DIN < 1 µM, N:P < 10, and Si:N > 1; P limitation if DRP < 0.1 µM, N:P > 22, and Si:P > 22; and Si limitation if DSi < 2 µM, Si:N < 1, and Si:P < 10 (Justíc et al. 1995).
The occurrence of nutrient limitation was evaluated using the responses of the phytoplankton community (using chlorophyll-a concentration) and specific functional groups (using abundance) to N and P enrichments, during microcosm experiments, using a two-way analysis of variance (ANOVA). The main effects (N, P) and interactions (NxP) were indicators of the occurrence and type of nutrient limitation, according to (Harpole et al. 2011; Lewis et al. 2020). (a) no limitation: non-significant main effect and non-significant interaction; (b) single nutrient limitation: one significant main effect (N or P) and non-significant interaction; (c) serial limitation: one significant main effect (N or P) and significant interaction; (d) simultaneous co-limitation: significant interaction with non-significant or two significant main effects (N and P); and (e) independent co-limitation: significant main effects (N and P) and non-significant interaction (Fig. 2).
Data Analyses
Two-way ANOVA was used to evaluate the effects of N and P amendments on the abundance of total phytoplankton and specific functional groups. N and P were used as factors and abundance at the end of each experiment as dependent variable. Data normality and homogeneity of variances were tested with Shapiro–Wilk and Levene’s tests, respectively. Differences in phytoplankton community structure across experimental treatments were assessed with one-way permutational multivariate analyses of variance (PERMANOVA) using 9999 unrestricted permutations of raw data. Data analyses were performed with IBM SPSS Statistics 28 and Primer v6 (with add-on for PERMANOVA +). All tests were considered at a significance level of 0.05.
Results
Initial Conditions and Potential Nutrient Limitation Based on Ambient Data
Abiotic and biotic conditions at the beginning of each experiment are depicted in Tables 1 and 2, for the inner and outer lagoon locations, respectively. At the inner lagoon, NP ratios indicated a potential nitrogen limitation of phytoplankton growth for all experiments, with starting N:P values systematically below 20 (Fig. 3). At the outer lagoon, ambient N:P at the beginning of the autumn and spring experiments were also indicative of N limitation; in the summer, N:P above 50 suggested P limitation, whereas in the winter, an intermediate N:P was indicative of colimitation (Fig. 3). However, when considering the combination of nutrient ratios and concentrations, N and P were never limiting, as DIN and DRP concentrations are always higher than 1 µM and 0.1 µM, respectively (Tables 1 and 2). Si was potentially limiting only in the outer lagoon, at the beginning of the autumn experiment, with DSi concentration lower than 2 µM, Si:N < 1, and Si:P < 10 (Table 2).
Application of the resource-ratio hypothesis to ambient nutrient concentrations of nitrogen (N) and phosphorus (P) in the Ria Formosa coastal lagoon at the beginning of each experiment, for the inner lagoon (open circles) and outer lagoon (black circles). N:P = 20 and N:P = 50 solid lines define three areas within the plot: N:P < 20 – N limitation; 20 < N:P < 50 – N and/or P limitation; N:P > 50 – P limitation (thresholds according to Guildford and Hecky 2000). Dashed line is N:P = 16. Letters at the right of each symbol denote the results of nutrient enrichment microcosm experiments: X, no limitation; N, single nitrogen limitation; SP, serial phosphorus limitation; C, colimitation; neg, negative effect
Regarding phytoplankton composition at the beginning of the experiments, the major contributors to phytoplankton abundance were nano- and pico-sized cells, namely autotrophic nanoflagellates, eukaryotic picophytoplankton, and Synechococcus-like cyanobacteria, for both sampling locations (Tables 1 and 2). Diatoms were mainly small centric species of the orders Thalassiosirales and Chaetocerotales. Pseudo-nitzschia and toxigenic dinoflagellates were never detected in numbers higher than alert levels (data not shown). Chlorophyll-a concentration at the beginning of the experiments varied between 0.7 and 4.5 µg L−1 at the inner lagoon and between 0.4 and 2.0 µg L−1 at the outer lagoon (Tables 1 and 2).
Experimental Evaluation of Nutrient Limitation Occurrence and Type
Nutrient enrichment experiments revealed potential nutrient limitation of phytoplankton growth at the inner lagoon across different functional groups and in all seasons except winter (Table 3). In the other seasons, diatoms were always limited, either by N only (spring) or simultaneously co-limited by N and P (summer and autumn); in the spring, N and NP additions led to 137% and 176% increases in diatom abundance, respectively, in relation to the control. The most widespread nutrient limitation was observed during summer, when the whole phytoplankton assemblage and several functional groups (diatoms and dinoflagellates) were simultaneously co-limited by N and P, and cyanobacteria showed serial P limitation. NP enrichment promoted a 233% increase in diatom abundance and a 492% increase in dinoflagellate abundance in relation to the control. Cyanobacteria abundance increased by 195% following NP addition. Eukaryotic picophytoplankton and other autotrophic nanoflagellates were never limited at the inner lagoon (Table 3, Figs. 4 and 5).
Abundance (cell L−1) of specific phytoplankton groups (rows) at the end of nutrient enrichment experiments in each experimental treatment (C, control; N, nitrogen addition; P, phosphorus addition; NP, nitrogen and phosphorus addition) at the inner lagoon. ANF, autotrophic nanoflagellates; EPP, eukaryotic picophytoplankton
At the outer lagoon, nutrient limitation was detected in all seasons except autumn, but different phytoplankton groups responded differently to nutrient amendments. In the winter, nutrient limitation was observed for the smaller-sized eukaryotic phytoplankton, with the occurrence of N limitation or simultaneous N and P co-limitation, with corresponding increases in abundance of 104% and 111%, respectively, in relation to the control. In the spring and summer, micro-sized phytoplanktonic groups, namely diatoms and dinoflagellates, were also nutrient-limited. During spring, simultaneous N and P co-limitation was detected for both groups, leading to increases in abundance of 710% and 30% for diatoms and dinoflagellates, respectively, in relation to the control. In the spring, N limitation was observed for the whole phytoplankton assemblage; N and NP additions promoted 150% and 200% increases in chlorophyll-a concentration, respectively, in relation to the control. In the summer, diatoms exhibited serial P limitation in the summer, with P additions leading to a 187% increase in diatom abundance (Table 3, Figs. 4 and 6).
Abundance (cell L−1) of specific phytoplankton groups (rows) at the end of nutrient enrichment experiments in each experimental treatment (C, control; N, nitrogen addition; P, phosphorus addition; NP, nitrogen and phosphorus addition) at the outer lagoon. ANF, autotrophic nanoflagellates; EPP, eukaryotic picophytoplankton
Negative significant responses to nutrient enrichment, whereby final abundance after nutrient enrichment was lower than initial abundance, were also observed for some functional groups in some experiments, but only at the outer lagoon. In this location, negative responses were frequently observed, for eukaryotic picophytoplankton (autumn and summer), dinoflagellates (winter and summer), cryptophytes (spring), and autotrophic nanoflagellates (summer) (Table 3).
Effects of Nutrient Enrichment on Phytoplankton Biomass and Community Structure
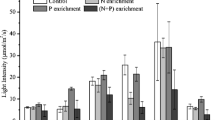
Nutrient enrichment promoted significant increases in phytoplankton biomass (measured as chlorophyll-a concentration) only in two of the experiments (Fig. 4). At the outer lagoon, during spring, chlorophyll-a concentration increased from 0.4 to 0.9 µg L−1 and 1.1 µg L−1 in treatments N and NP, respectively. For the inner lagoon, during summer, chlorophyll-a concentration increased from 4.6 to 13.4 µg L−1 and 27.4 µg L−1 in treatments N and NP, respectively.
Despite the significant effects of N and P enrichment on several phytoplankton functional groups, PERMANOVA did not detect significant changes in the overall structure of phytoplankton assemblages (based on the abundance of functional groups) across experimental treatments for most experiments. The only exception was observed at the outer lagoon for the autumn experiment, in which P and NP amendments were associated with a significant relative increase in cyanobacteria abundance, and a decrease in eukaryotic picophytoplankton abundance in relation to the control (p = 0.030) (Fig. 7).
Phytoplankton composition at the end of each nutrient enrichment experiment (based on abundance), with the relative contribution of diatoms, eukaryotic picophytoplankton (euk picophyto), cyanobacteria, and dinoflagellates + cryptophytes + other autotrophic nanoflagellates (flagellates). The four areas within each plot correspond to the four experimental treatments: control, LPxLN; N, LPxHN; P, HPxLN; NP, HPxHN (LN, low nitrogen; LP, low phosphorus; HN, high nitrogen; HP, high phosphorus)
Discussion
Effects of Nutrient Enrichment on Phytoplankton
The nutrient enrichment experiments conducted in two distinct locations of the Ria Formosa coastal lagoon throughout an annual cycle showed that simultaneous co-limitation by nitrogen and phosphorus was the most common type of nutrient limitation for phytoplankton, contrary to our working hypothesis that put a greater emphasis on nitrogen. However, the occurrence and nature of nutrient limitation of phytoplankton growth varied depending on the phytoplankton functional groups, season, and lagoon location.
This study is the first to detect co-limitation by N and P for phytoplankton in the Ria Formosa. Previous experimental studies only referred nitrogen as the most likely potentially limiting nutrient, although significant nutrient (N, P, and Si) consumption after nutrient enrichment was detected and attributed to luxury consumption or delayed growth responses (Loureiro et al. 2005; Domingues et al. 2015, 2017a). The occurrence of synergistic interactions between limiting resources is widespread across aquatic ecosystems (Elser et al. 2007), although it contradicts Liebig’s Law of the Minimum that posits that only one resource will be limiting at any given time. However, in the last decades this notion of single resource limitation has evolved to a more complex view of limitation by multiple resources, including not only nutrients, but also light (Arrigo 2005). In the Ria Formosa coastal lagoon, light limitation seldom occurs, being restricted to deeper lagoon locations and only during winter months (Domingues et al. 2017a). Indeed, nutrients, rather than light, have been referred as main factors controlling phytoplankton growth in many coastal lagoons. For instance, in the Coorang lagoon, Australia, and in Patos lagoon, Brazil, nutrients are the main drivers of phytoplankton variability and species distribution (Mendes et al. 2017; Hemraj et al. 2017), whereas in the Indian River, USA, nutrients were shown to increase the potential for bloom development (Phlips et al. 2021).
In the Ria Formosa coastal lagoon, co-limitation by N and P occurred in the form of simultaneous co-limitation, whereby growth responses are observed when both limiting resources are added simultaneously (Harpole et al. 2011). This response was observed at the inner and outer lagoon locations, and it was more frequent in the spring and summer, periods when nutrient concentrations are typically lower (nitrate < 4.7 µM, phosphate < 0.6 µM) and light is not limiting (Barbosa 2010; Domingues et al. 2015). Co-limitation of phytoplankton growth by N and P is commonly observed during specific time periods, not only in coastal ecosystems (Zohary et al. 2005; Leruste et al. 2019, 2021; Serre-Fredj et al. 2022), but also in freshwater (Bratt et al. 2020; Lewis et al. 2020). In freshwater systems, the deeply rooted dogma of P-limited phytoplankton growth is now considered overgeneralised, and both field and experimental data show that higher algal growth is associated not with single inputs of P or N, but rather with inputs of both N and P (Paerl et al. 2016). In a Mediterranean coastal lagoon, co-limitation by N and P was observed in the summer for all phytoplankton size groups, and in the spring only for nano-sized groups (Leruste et al. 2019); in this lagoon, co-limitation by N and P was a frequent occurrence, despite high N:P ratios (Leruste et al. 2021). Co-limitation by N and Si was also observed, for instance, in the Curonian lagoon (Baltic coast), associated with a shift from a diatom-based to cyanobacteria-dominated community (Vybernaite-Lubiene et al. 2017). The old paradigm of N limitation in coastal ecosystems and P limitation in freshwater has been surpassed, and co-limitation by N and P is a common condition in heterogeneous phytoplankton communities due to taxa-specific nutritional demands (Danger et al. 2008; Bannon et al. 2022).
Although co-limitation by N and P was predominant in the Ria Formosa coastal lagoon, other types of nutrient limitation were also detected occasionally, including single nutrient limitation, the typical resource limitation postulated by Liebig’s Law. Single N limitation was limited to the spring period (diatoms at inner lagoon), and single P limitation occurred only during summer (cyanobacteria at inner, and diatoms at outer lagoon). Occasional serial P limitation was also detected; serial limitation occurs when phytoplankton responds to a single resource (in this case, P) when added individually, but synergistic responses to two resources (P and N) are also observed when added together (Harpole et al. 2011). Single limitation by either N or P is also observed in other coastal ecosystems across the globe. For instance, phytoplankton in a tributary of the Indian River Lagoon, USA, clearly responded to additions of N, but P additions had no effects, indicating single limitation by N (Lin et al. 2008). On the contrary, in Moreton Bay, Australia, P is the most probable single limiting nutrient, as benthic N2-fixation is high, overcoming any potential N limitation (Wulff et al. 2011).
Regarding specific phytoplankton groups, diatoms were the most sensitive group to nutrient enrichment (limitation in 62.5% experiments), particularly when N and P were added simultaneously, thus supporting our working hypothesis. As an r-strategist opportunistic group, usually adapted to well mixed and nutrient-enriched waters (high half-saturation constants for nutrient uptake; Weithoff and Beisner 2019), diatoms are able to take up and utilise new nutrients, such as nitrate, for growth, quickly increasing their biomass and often leading to blooms (Glibert 2016; Wasmund et al. 2017).
Dinoflagellates responses to nutrient enrichment revealed nutrient limitation at both lagoon locations, but in different seasons. Simultaneous co-limitation by N and P was observed in spring at the outer lagoon and in the summer at the inner lagoon. Dinoflagellate development is commonly associated with silica depletion, high water temperature, and regenerated ammonium inputs (Maier et al. 2012; Glibert et al. 2016). In contrast, spring and summer conditions in the Ria Formosa lagoon are characterised by non-limiting silica and low ammonium concentrations, favouring diatoms over dinoflagellates. However, dinoflagellates possess a series of life traits, such as motility, mixotrophy, or allelopathy (Smayda and Trainer 2010), that allow them to thrive in an array of nutritional conditions, which could explain the results observed in the enrichment experiments.
Prokaryotic and eukaryotic picophytoplankton were seldom limited by nutrients. Pico-sized Synechococcus-like cyanobacteria were serially limited by P only in the summer at the inner lagoon, whereas eukaryotic picophytoplankton showed co-limitation by N and P only in the winter at the outer lagoon. Overall, picophytoplankton are well adapted to low nutrient concentrations are more efficient at obtaining and utilising nutrients for growth (Wang et al. 2022). Nutrients usually have a rather weak role on the abundance of picoprokaryotes, whereas picoeukaryotes are positively related to nutrient concentrations (Agusti et al. 2019; Mouriño-Carballido et al. 2016).
Previous modelling (Rodrigues et al. 2021) and observational approaches (Rosa et al. 2022; Oduor et al. 2023) have suggested that silicon is a limiting nutrient in the Ria Formosa lagoon, based on half-saturation constants or the Redfield ratio. However, these metrics are not the most suitable to assess actual nutrient limitation of phytoplankton growth. Half-saturation constants are obtained under equilibrium conditions and vary temporally, spatially, intra- and inter-specifically (Domingues et al. 2005), and the cellular stoichiometry for individual algal species deviate significantly from the Redfield ratio (Garcia et al. 2018). In addition, when phytoplankton is limited by silicon, only diatoms and a few other Si-consuming organisms (e.g. silicoflagellates) are limited by silicon; other, non-Si-consuming phytoplankton, are not limited by Si, but rather by other resources. Furthermore, previous nutrient enrichment bioassays conducted in the Ria Formosa lagoon did not find any evidence of Si limitation of diatom growth. In these experiments, significant silicon consumption was observed after Si enrichment, but it did not result in enhanced diatom growth (Domingues et al. 2015, 2017a). This luxury consumption of Si by diatoms only occurs under non-limiting Si levels (Revilla and Weissing 2008), and is used for wall synthesis, resulting in thicker walls (Martin-Jézéquel et al. 2000).
Nutrient enrichment also promoted negative growth responses for specific phytoplankton groups, whereby the final abundance/biomass after nutrient enrichment was lower than the initial abundance/biomass. These negative responses are quite common (e.g. (Bratt et al. 2020) but rarely discussed in nutrient limitation studies (Harpole et al. 2011). Negative growth responses to nutrient addition were detected only at the outer lagoon, but for all seasons (more intensively summer) and functional groups, except diatoms. It has been suggested that these reductions in abundance/biomass may be due to stoichiometric constraints, particularly when N and P are added alone, but not when they are added together (Harpole et al. 2011). This could explain the negative responses observed in some of our experiments (e.g. autotrophic nanoflagellates and eukaryotic picophytoplankton in the summer, and cryptophytes in the spring). Other possible explanations could be competition with heterotrophic prokaryotes, and increased mortality (e.g. viral lysis, herbivory). Negative growth responses consistent with potential toxicity effects are much rarer in nutrient enrichment experiments (Harpole et al. 2011).
Despite significant growth responses to nutrient enrichment, the relative proportion of different phytoplankton groups did not reflect, for most experiments, the enhanced growth rates observed. The exception was a significant increase in the relative contribution of cyanobacteria and a decrease in eukaryotic picophytoplankton in the autumn experiment at the outer lagoon. Given that the relative composition of phytoplankton functional groups was based on abundance, and pico-sized groups are present in much larger numbers (106 cell L−1) in relation to microphytoplankton (103—105 cell L−1), increases in net growth rates of larger phytoplankton would not be evident in the relative composition of the whole assemblage.
Assessment of Nutrient Limitation: Ambient (Observational) Data Versus Experimental Data
Evaluating nutrient limitation is essential for the assessment of phytoplankton responses to nutrient over-enrichment, and nutrient addition bioassays are the dominant experimental approaches used to determine nutrient limitation of phytoplankton growth (Ren et al. 2009). Our nutrient enrichment experiments produced a wide range of responses, that suggested single N-limitation, serial P-limitation, N and P co-limitation, and even negative growth responses to enrichment. This variability in phytoplankton responses hampers our ability to classify the nature of phytoplankton limitation in the Ria Formosa as N- or P- or co-limited, and to predict ecosystem responses to nutrient enrichment. In the absence of experimental evaluations of nutrient limitation, assessments typically rely on nutrient stoichiometry. However, N:P ratios (ambient data) for the Ria Formosa did not correspond to the outcome of nutrient enrichment experiments. For instance, N:P ratio was always indicative of potential N limitation in the inner lagoon, but simultaneous co-limitation by N and P was the most common result of nutrient enrichment experiments. In the outer lagoon, potential nutrient limitation alternated between N, P, and co-limitation, but, in most cases, the potential limitations inferred from nutrient ratios were not compatible with experimental approaches. In addition, the combination of nutrient ratios and absolute concentrations to evaluate potential nutrient limitation was also not consistent with experimental data. This discrepancy can be explained by nutrient utilisation traits that vary intra- and interspecifically, such as half-saturation constants and uptake affinity (Edwards et al. 2012), luxury consumption of non-limiting nutrients (Hodapp et al. 2019), mixotrophy (Flynn et al. 2019), among others.
As in most marine open ocean and coastal ecosystems, nitrogen has been frequently referred as the most probable limiting nutrient of phytoplankton growth in the Ria Formosa coastal lagoon (Cravo et al. 2014; Domingues et al. 2015, 2017a). It is not clear whether co-limitation was also frequent in this ecosystem or if the N and P co-limitation is a new occurrence. It has been suggested that single nutrient limitation is more common in anthropogenically-impacted, nutrient-rich systems, whereas co-limitation is more likely in oligotrophic systems (Moss et al. 2013). Additionally, a shift from N limitation to P limitation or N and P co-limitation has been observed in ecosystems undergoing processes of oligotrophication (Derolez et al. 2020) and can favour the replacement of fast-growing diatoms by harmful dinoflagellates (Yamamoto 2003). Indeed, a generalised improvement in water quality has been detected in recent years, in relation to the early 2000’s, due to the upgrade of wastewater treatment systems (Rosa et al. 2022). Recent observational and experimental studies suggest that the Ria Formosa is not in a poor condition regarding eutrophication (Domingues et al. 2017a; Cravo et al. 2022). These studies have not identified neither persistent large inputs of N and P to the Ria Formosa, nor unbalanced N:P ratios. In our study, chlorophyll-a values at the beginning and end of the nutrient enrichment experiments were below the reference value of 5.3 µg L−1 and below the threshold value for the good/moderate class (12 µg L−1) (Brito et al. 2012). The exception was observed during the summer experiment at the inner lagoon, when a single addition of N and simultaneous additions of N and P led to increases in chlorophyll-a from 4.6 µg L−1 at the beginning of incubation to 13.4 µg L−1 (N) and 27.4 µg L−1 (NP) after 24 h. These results suggest that the Ria Formosa is still susceptible to nutrient over-enrichment, particularly during the productive season, when light in the water column is not limiting phytoplankton growth (Domingues et al. 2015), despite the recent increase in water quality (see Rosa et al. 2022).
When using an experimental-based strategy, chlorophyll-a concentration is still the preferred phytoplankton-related metric to evaluate eutrophication, being extensively used to evaluate nutrient limitation across ecosystems (Elser et al. 2007; Harpole et al. 2011) due to its time and cost-effectiveness and high reproducibility (Domingues et al. 2008). However, the use of chlorophyll-a may not capture responses of specific phytoplankton groups, particularly smaller-sized prokaryotes and pico-eukaryotes, and less abundant (sometimes harmful/toxigenic) dinoflagellates. The use of phytoplankton pigment-based growth responses as an alternative or complement to chlorophyll-a also preclude the detection of phytoplankton species-specific responses, only observable through the use of a microscopy-based technique (Cira et al. 2016). The continued use of microscopic identification and enumeration in phytoplankton research is fundamental to properly evaluate true community and taxa-specific growth responses to environmental perturbations, including nutrient enrichment (Cira et al. 2016; McQuatters-Gollop et al. 2017).
Conclusions
Identifying the limiting nutrient for phytoplankton growth is essential for the evaluation of eutrophication in coastal ecosystems. In the Ria Formosa coastal lagoon, the most frequent type of nutrient limitation throughout an annual cycle was simultaneous co-limitation by nitrogen and phosphorus, but single nitrogen limitation and serial phosphorus limitation were also observed. Diatoms were the most frequently limited phytoplankton groups, and community biomass responses, the most common metric used to evaluate the effects of nutrient enrichment, did not capture group-specific responses. Potential nutrient limitation inferred by nutrient molar ratios, such as the Redfield ratio, and other indicators based on stoichiometry and absolute concentrations, was not congruent with nutrient limitation assessed by nutrient enrichment microcosm experiments. Experimental results also contradict previous findings based on observational approaches that put a greater emphasis on silicon as the limiting nutrient in the Ria Formosa lagoon. Overall, our findings demonstrate that phytoplankton responses to nutrient enrichment is variable across seasons, locations, and phytoplankton functional groups, and that phytoplankton taxonomy is critical to evaluate these responses. Therefore, eutrophication assessments should not be based on broad indicators (like chlorophyll-a concentration) but rather on metrics that reflect the intricacies of these dynamic ecosystems.
Data Availability
Data is available upon request to the corresponding author.
References
Agusti, S., L.M. Lubián, E. Moreno-Ostos, M. Estrada, and C.M. Duarte. 2019. Projected changes in photosynthetic picoplankton in a warmer subtropical ocean. Frontiers in Marine Science 5. https://doi.org/10.3389/fmars.2018.00506.
Anderson, D.M., A.D. Cembella, and G.M. Hallegraeff. 2012. Progress in understanding harmful algal blooms (HABs): Paradigm shifts and new technologies for research, monitoring and management. Annual Review of Marine Science 4: 143–176. https://doi.org/10.1146/annurev-marine-120308-081121.Progress.
Arias, P.A., N. Bellouin, E. Coppola, R.G. Jones, G. Krinner, J. Marotzke, V. Naik. et al. 2021. Technical summary. In Climate Change 2021: The Physical Science Basis. Contribution of Working Group I to the Sixth Assessment Report of the Intergovernmental Panel on Climate Change, ed. V. Masson-Delmotte, P. Zhai, A. Pirani, S. L. Connors, C. Péan, S. Berger, N. Caud, et al., 33–144. Cambridge, United Kingdom and New York, NY, USA: Cambridge University Press. https://doi.org/10.1017/9781009157896.002.
Arrigo, K.R. 2005. Marine microorganisms and global nutrient cycles. Nature 437: 349–355. https://doi.org/10.1038/nature04159.
Bannon, C., I. Rapp, and E.M. Bertrand. 2022. Community interaction co-limitation: Nutrient limitation in a marine microbial community context. Frontiers in Microbiology 13: 1–16. https://doi.org/10.3389/fmicb.2022.846890.
Barbosa, A.B. 2010. Seasonal and interanual variability of planktonic microbes in a mesotidal coastal lagoon (Ria Formosa , SE Portugal). Impact of climatic changes and local human influences. In Coastal Lagoons: Critical Habitats of Environmental Change, ed. M. J. Kennish and H. W. Paerl, 334–366. CRC Press.
Beardall, J., E. Young, and S. Roberts. 2001. Approaches for determining phytoplankton nutrient limitation. Aquatic Sciences 63: 44–69. https://doi.org/10.1007/PL00001344.
Bratt, A.R., J.C. Finlay, J.R. Welter, B.A. Vculek, and R.E. Van Allen. 2020. Co-limitation by N and P characterizes phytoplankton communities across nutrient availability and land use. Ecosystems 23. Springer US: 1121–1137. https://doi.org/10.1007/s10021-019-00459-6.
Brito, A.C., T. Quental, T.P. Coutinho, M.A.C. Branco, M. Falcão, A. Newton, J. Icely, and T. Moita. 2012. Phytoplankton dynamics in southern Portuguese coastal lagoons during a discontinuous period of 40 years: An overview. Estuarine, Coastal and Shelf Science 110: 147–156. https://doi.org/10.1016/j.ecss.2012.04.014.
Burson, A., M. Stomp, E. Greenwell, J. Grosse, and J. Huisman. 2018. Competition for nutrients and light: Testing advances in resource competition with a natural phytoplankton community. Ecology 99: 1108–1118. https://doi.org/10.1002/ecy.2187.
Cira, E.K., H.W. Paerla, and M.S. Wetza. 2016. Effects of nitrogen availability and form on phytoplankton growth in a eutrophied estuary (Neuse River Estuary, NC, USA). PLoS ONE 11: 1–15. https://doi.org/10.1371/journal.pone.0160663.
Cravo, A, A.B. Barbosa, C. Correia, A. Matos, S. Caetano, M. J. Lima, and J. Jacob. 2022. Unravelling the effects of treated wastewater discharges on the water quality in a coastal lagoon system (Ria Formosa, South Portugal): relevance of hydrodynamic conditions. Marine Pollution Bulletin 174. Elsevier Ltd: 113296. https://doi.org/10.1016/j.marpolbul.2021.113296.
Cravo, A., S. Cardeira, C. Pereira, M. Rosa, P. Alcântara, M. Madureira, F. Rita, C. Correia, A. Rosa, and J. Jacob. 2019. Nutrients and chlorophyll-a exchanges through an inlet of the Ria Formosa Lagoon, SW Iberia during the productive season – unravelling the role of the driving forces. Journal of Sea Research 144. Elsevier: 133–141. https://doi.org/10.1016/j.seares.2018.12.001.
Cravo, A., S. Cardeira, C. Pereira, M. Rosa, P. Alcântara, M. Madureira, F. Rita, J. Luis, and J. Jacob. 2014. Exchanges of nutrients and chlorophyll a through two inlets of Ria Formosa, South of Portugal, during coastal upwelling events. Journal of Sea Research 93: 63–74. https://doi.org/10.1016/j.seares.2014.04.004.
Cravo, A., D. Fernandes, T. Damião, C. Pereira, and M.P. Reis. 2015. Determining the footprint of sewage discharges in a coastal lagoon in South-Western Europe. Marine Pollution Bulletin 96. Elsevier Ltd: 197–209. https://doi.org/10.1016/j.marpolbul.2015.05.029.
Cravo, A., C. Ferreira, and J. Jacob. 2018. Water quality improvement in Ria Formosa since the early 2000? In Sanitation Approaches and Solutions and The Sustainable Development Goals, ed. J. Saldanha Matos and M. J. Rosa, 307–322. ERSAR – Entidade Reguladora dos Serviços de Águas e Serviços, EWA – European Water Association, APESB – Associação Portuguesa de Engenharia Sanitária e Ambiental.
Danger, M., T. Daufresne, F. Lucas, S. Pissard, and G. Lacroix. 2008. Does Liebig’s law of the minimum scale up from species to communities? Oikos 117: 1741–1751. https://doi.org/10.1111/j.1600-0706.2008.16793.x.
Derolez, V., D. Soudant, N. Malet, C. Chiantella, M. Richard, E. Abadie, C. Aliaume, and B. Bec. 2020. Two decades of oligotrophication: evidence for a phytoplankton community shift in the coastal lagoon of Thau (Mediterranean Sea, France). Estuarine, Coastal and Shelf Science 241. https://doi.org/10.1016/j.ecss.2020.106810.
Domingues, R.B., T.P. Anselmo, A.B. Barbosa, U. Sommer, and H.M. Galvão. 2011. Nutrient limitation of phytoplankton growth in the freshwater tidal zone of a turbid, Mediterranean estuary. Estuarine, Coastal and Shelf Science 91: 282–297. https://doi.org/10.1016/j.ecss.2010.10.033.
Domingues, R.B., A.B. Barbosa, and H.M. Galvão. 2008. Constraints on the use of phytoplankton as a biological quality element within the Water Framework Directive in Portuguese waters. Marine Pollution Bulletin 56: 1389–1395. https://doi.org/10.1016/j.marpolbul.2008.05.006.
Domingues, R.B., C.C. Guerra, A.B. Barbosa, and H.M. Galvão. 2015. Are nutrients and light limiting summer phytoplankton in a temperate coastal lagoon? Aquatic Ecology 49: 127–146. https://doi.org/10.1007/s10452-015-9512-9.
Domingues, R.B., C.C. Guerra, A.B. Barbosa, and H.M. Galvão. 2017a. Will nutrient and light limitation prevent eutrophication in an anthropogenically-impacted coastal lagoon? Continental Shelf Research 141. Elsevier Ltd: 11–25. https://doi.org/10.1016/j.csr.2017.05.003.
Domingues, R.B., C.C. Guerra, H.M. Galvão, V. Brotas, and A. Barbosa. 2017b. Short-term interactive effects of ultraviolet radiation, carbon dioxide and nutrient enrichment on phytoplankton in a shallow coastal lagoon. Aquatic Ecology 51: 91–105. https://doi.org/10.1007/s10452-016-9601-4.
Domingues, R.B., A. Barbosa, and H. Galvão. 2005. Nutrients, light and phytoplankton succession in a temperate estuary (the Guadiana, south-western Iberia ). Estuarine, Coastal and Shelf Science 64: 249–260. https://doi.org/10.1016/j.ecss.2005.02.017.
Edwards, K.F., M.K. Thomas, C.A. Klausmeier, and E. Litchman. 2012. Allometric scaling and taxonomic variation in nutrient utilization traits and maximum growth rate of phytoplankton. Limnology and Oceanography 57: 554–566. https://doi.org/10.4319/lo.2012.57.2.0554.
Elser, J.J., M.E.S. Bracken, E.E. Cleland, D.S. Gruner, W.S. Harpole, H. Hillebrand, J.T. Ngai, E.W. Seabloom, J.B. Shurin, and J.E. Smith. 2007. Global analysis of nitrogen and phosphorus limitation of primary producers in freshwater, marine and terrestrial ecosystems. Ecology Letters 10: 1135–1142. https://doi.org/10.1111/j.1461-0248.2007.01113.x.
Flynn, K.J., A. Mitra, K. Anestis, A.A. Anschütz, A. Calbet, G.D. Ferreira, N. Gypens, et al. 2019. Mixotrophic protists and a new paradigm for marine ecology: Where does plankton research go now? Journal of Plankton Research 41: 375–391. https://doi.org/10.1093/plankt/fbz026.
Garcia, N.S., J. Sexton, T. Riggins, J. Brown, M.W. Lomas, and A.C. Martiny. 2018. High variability in cellular stoichiometry of carbon, nitrogen, and phosphorus within classes of marine eukaryotic phytoplankton under sufficient nutrient conditions. Frontiers in Microbiology 9: 1–10. https://doi.org/10.3389/fmicb.2018.00543.
Glibert, P.M., and M.A. Burford. 2017. Globally changing nutrient loads and harmful algal blooms: Recent advances, new paradigms, and continuing challenges. Oceanography 30: 58–69. https://doi.org/10.5670/oceanog.2017.110.
Glibert, P.M., C.A. Heil, F.P. Wilkerson, and R.C. Dugdale. 2018. Nutrients and harmful algal blooms: dynamic kinetics and flexible nutrition. In Global Ecology and Oceanography of Harmful Algal Blooms, ed. Patricia M. Glibert, E. Berdalet, M. A. Burford, G. C. Pitcher, and M. Zhou, 93–112. Springer.
Glibert, P.M. 2016. Margalef revisited: a new phytoplankton mandala incorporating twelve dimensions, including nutritional physiology. Harmful Algae 55. Elsevier B.V.: 25–30. https://doi.org/10.1016/j.hal.2016.01.008.
Glibert, P.M., F.P. Wilkerson, R.C. Dugdale, J.A. Raven, C.L. Dupont, P.R. Leavitt, A.E. Parker, J.M. Burkholder, and T.M. Kana. 2016. Pluses and minuses of ammonium and nitrate uptake and assimilation by phytoplankton and implications for productivity and community composition, with emphasis on nitrogen-enriched conditions. Limnology and Oceanography 165–197. https://doi.org/10.1002/lno.10203
Grasshoff, K., M. Ehrhardt, and K. Kremling. 1999. Methods of seawater analysis. third. Weinheim: WILEY‐VCH Verlag GmbH. https://doi.org/10.1002/9783527613984.
Guildford, S.J., and R.E. Hecky. 2000. Total nitrogen, total phosphorus, and nutrient limitation in lakes and oceans: Is there a common relationship? Limnology and Oceanography 45: 1213–1223. https://doi.org/10.4319/lo.2000.45.6.1213.
Haas, L.W. 1982. Improved epifluorescence microscopy for observing planktonic micro-organisms. Annales De L’institut Oceanographique 58: 261–266.
Harpole, W.S., J.T. Ngai, E.E. Cleland, E.W. Seabloom, E.T. Borer, M.E.S. Bracken, J.J. Elser, et al. 2011. Nutrient co-limitation of primary producer communities. Ecology Letters 14: 852–862. https://doi.org/10.1111/j.1461-0248.2011.01651.x.
Heisler, J., P.M. Glibert, J.M. Burkholder, D.M. Anderson, W. Cochlan, W.C. Dennison, Q. Dortch, et al. 2008. Eutrophication and harmful algal blooms: A scientific consensus. Harmful Algae 8: 3–13. https://doi.org/10.1016/j.hal.2008.08.006.
Hemraj, D.A., M.A. Hossain, Q. Ye, J.G. Qin, and S.C. Leterme. 2017. Plankton bioindicators of environmental conditions in coastal lagoons. Estuarine, Coastal and Shelf Science 184: 102–114. https://doi.org/10.1016/j.ecss.2016.10.045.
Hodapp, D., H. Hillebrand, and M. Striebel. 2019. “Unifying” the concept of resource use efficiency in ecology. Frontiers in Ecology and Evolution 6: 1–14. https://doi.org/10.3389/fevo.2018.00233.
Jacob, J., C. Correia, A.F. Torres, G. Xufre, A. Matos, C. Ferreira, M.P. Reis, et al. 2020. Impacts of decommissioning and upgrading urban wastewater treatment plants on the water quality in a shellfish farming coastal lagoon (Ria Formosa, South Portugal). Journal of Coastal Research 95: 45. https://doi.org/10.2112/SI95-009.1.
Jeppesen, E., B. Moss, H. Bennion, L. Carvalho, L. Demeester, H. Feuchtmayr, N. Friberg, et al. 2010. Interaction of climate change and eutrophication. In Climate Change Impacts on Freshwater Ecosystems, ed. M. Kernan, R. Battarbee, and B. Moss, 119–151. Blackwell Publishing.
Justíc, D., N.N. Rabalais, R.E. Turner, and Q. Dortch. 1995. Changes in nutrient structure of river dominated coastal waters: Stoichiometric nutrient balance and its consequences. Estuarine, Coastal and Shelf Science 40: 339–356.
Kennish, M., and H. Paerl. 2010. Coastal lagoons: critical habitats of environmental change. In Coastal Lagoons: Critical Habitats of Environmental Change, ed. M. Kennish and H. Paerl, 1–15. CRC Press.
Leruste, A., V. Pasqualini, M. Garrido, N. Malet, R. De Wit, and B. Bec. 2019. Physiological and behavioral responses of phytoplankton communities to nutrient availability in a disturbed Mediterranean coastal lagoon. Estuarine, Coastal and Shelf Science 219. Elsevier: 176–188. https://doi.org/10.1016/j.ecss.2019.02.014.
Leruste, A., M. Garrido, N. Malet, B. Bec, R. De Wit, P. Cecchi, and V. Pasqualini. 2021. Impact of nutrient availability on the trophic strategies of the planktonic protist communities in a disturbed Mediterranean coastal lagoon. Hydrobiologia 848: 1101–1119. https://doi.org/10.1007/s10750-021-04517-w.
Lewis, A.S.L., B.S. Kim, H.L. Edwards, H.L. Wander, C.M. Garfield, H.E. Murphy, N.D. Poulin, et al. 2020. Prevalence of phytoplankton limitation by both nitrogen and phosphorus related to nutrient stoichiometry, land use, and primary producer biomass across the northeastern United States. Inland Waters 10. Taylor & Francis: 42–50. https://doi.org/10.1080/20442041.2019.1664233.
Lima, M.J., P. Relvas, and A.B. Barbosa. 2022. Variability patterns and phenology of harmful phytoplankton blooms off southern Portugal: looking for region-specific environmental drivers and predictors. Harmful Algae 116. Elsevier B.V.: 102254. https://doi.org/10.1016/j.hal.2022.102254.
Lin, Y., Z. He, Y. Yang, P.J. Stoffella, E.J. Phlips, and C.A. Powell. 2008. Nitrogen versus phosphorus limitation of phytoplankton growth in Ten Mile Creek, Florida, USA. Hydrobiologia 605: 247–258. https://doi.org/10.1007/s10750-008-9360-x.
Loureiro, S., A. Newton, and J. Icely. 2005. Effects of nutrient enrichments on primary production in the Ria Formosa coastal lagoon (Southern Portugal). Hydrobiologia 550: 29–45. https://doi.org/10.1007/s10750-005-4357-1.
Loureiro, S., A. Newton, and J. Icely. 2006. Boundary conditions for the European Water Framework Directive in the Ria Formosa lagoon, Portugal (physico-chemical and phytoplankton quality elements). Estuarine, Coastal and Shelf Science 67: 382–398. https://doi.org/10.1016/j.ecss.2005.11.029.
Maier, G., G.A. Glegg, A.D. Tappin, and P.J. Worsfold. 2012. A high resolution temporal study of phytoplankton bloom dynamics in the eutrophic Taw Estuary (SW England). Science of the Total Environment 434: 228–239.
Martin-Jézéquel, V., M. Hildebrand, and M.A. Brzezinski. 2000. Silicon metabolism in diatoms: Implications for growth. Journal of Phycology 36: 821–840.
McQuatters-Gollop, A., D.G. Johns, E. Bresnan, J. Skinner, I. Rombouts, R. Stern, A. Aubert, M. Johansen, J. Bedford, and A. Knights. 2017. From microscope to management: the critical value of plankton taxonomy to marine policy and biodiversity conservation. Marine Policy 83. Elsevier Ltd: 1–10. https://doi.org/10.1016/j.marpol.2017.05.022.
Mendes, C.R.B., C. Odebrecht, V.M. Tavano, and P.C. Abreu. 2017. Pigment-based chemotaxonomy of phytoplankton in the Patos Lagoon estuary (Brazil) and adjacent coast. Marine Biology Research 13: 22–35. https://doi.org/10.1080/17451000.2016.1189082.
Moss, B., E. Jeppesen, M. Søndergaard, T.L. Lauridsen, and Z. Liu. 2013. Nitrogen, macrophytes, shallow lakes and nutrient limitation: Resolution of a current controversy? Hydrobiologia 710 (1): 3–21. https://doi.org/10.1007/s10750-012-1033-0.
Mouriño-Carballido, B., E. Hojas, P. Cermeño, P. Chouciño, B. Fernández-Castro, M. Latasa, E. Marañón, X. Morán, and M. Vidal. 2016. Nutrient supply controls picoplankton community structure during three contrasting seasons in the northwestern Mediterranean Sea. Marine Ecology Progress Series 543: 1–19. https://doi.org/10.3354/meps11558.
Newton, A., A.C. Brito, J.D. Icely, V. Derolez, I. Clara, S. Angus, G. Schernewski, et al. 2018. Assessing, quantifying and valuing the ecosystem services of coastal lagoons. Journal for Nature Conservation 44: 50–65. https://doi.org/10.1016/j.jnc.2018.02.009.
Newton, A., J. Icely, S. Cristina, A. Brito, A.C. Cardoso, F. Colijn, S.D. Riva, et al. 2014. An overview of ecological status, vulnerability and future perspectives of European large shallow, semi-enclosed coastal systems, lagoons and transitional waters. Estuarine, Coastal and Shelf Science 140: 95–122. https://doi.org/10.1016/j.ecss.2013.05.023.
Newton, A., J. Icely, S. Cristina, G.M.E. Perillo, R.E. Turner, D. Ashan, S. Cragg, et al. 2020. Anthropogenic, direct pressures on coastal wetlands. Frontiers in Ecology and Evolution 8: 1–29. https://doi.org/10.3389/fevo.2020.00144.
Nogueira, P., R.B. Domingues, and A.B. Barbosa. 2014. Are microcosm volume and sample pre-filtration relevant to evaluate phytoplankton growth? Journal of Experimental Marine Biology and Ecology 461. Elsevier B.V.: 323–330. https://doi.org/10.1016/j.jembe.2014.09.006.
Oduor, N.A., S.C. Cristina, and P. Costa. 2023. Sources of anthropogenic nutrients and their implications on nutrient chemistry and ecological conditions of Ria Formosa lagoon, Portugal. Regional Studies in Marine Science 61. https://doi.org/10.1016/j.rsma.2023.102843
Paerl, H.W., J.T. Scott, M.J. McCarthy, S.E. Newell, W.S. Gardner, K.E. Havens, D.K. Hoffman, S.W. Wilhelm, and W.A. Wurtsbaugh. 2016. It takes two to tango: When and where dual nutrient (N & P) reductions are needed to protect lakes and downstream ecosystems. Environmental Science and Technology 50 (20): 10805–10813. https://doi.org/10.1021/acs.est.6b02575.
Parsons, T.R., Y. Maita, and C.M. Lalli. 1984. A manual of chemical and biological methods for seawater analysis. Pergamon Press.
Phlips, E.J., S. Badylak, N.G. Nelson, L.M. Hall, C.A. Jacoby, M.A. Lasi, J.C. Lockwood, and J. D. Miller. 2021. Cyclical patterns and a regime shift in the character of phytoplankton blooms in a restricted sub-tropical lagoon, Indian River Lagoon, Florida, United States. Frontiers in Marine Science 8: 730934. https://doi.org/10.3389/fmars.2021.730934.
Relvas, P., E.D. Barton, J. Dubert, P.B. Oliveira, Á. Peliz, J.C.B. da Silva, and A.M.P. Santos. 2007. Physical oceanography of the western Iberia ecosystem: Latest views and challenges. Progress in Oceanography 74: 149–173. https://doi.org/10.1016/j.pocean.2007.04.021.
Ren, L., N.N. Rabalais, R.E. Turner, W. Morrison, and W. Mendenhall. 2009. Nutrient limitation on phytoplankton growth in the Upper Barataria Basin, Louisiana: Microcosm bioassays. Estuaries and Coasts 32: 958–974. https://doi.org/10.1007/s12237-009-9174-8.
Revilla, T., and F.J. Weissing. 2008. Nonequilibrium coexistence in a competition model with nutrient storage. Ecology 89: 865–877.
Rodrigues, M., A. Rosa, A. Cravo, J. Jacob, and A. B. Fortunato. 2021. Effects of climate change and anthropogenic pressures in the water quality of a coastal lagoon (Ria Formosa, Portugal). Science of The Total Environment 780: 146311. https://doi.org/10.1016/j.scitotenv.2021.146311.
Rosa, A., A. Cravo, J. Jacob, and C. Correia. 2022. Water quality of a southwest Iberian coastal lagoon: spatial and temporal variability. Continental Shelf Research 245. Elsevier Ltd: 104804. https://doi.org/10.1016/j.csr.2022.104804.
Serre-Fredj, L., L. Chasselin, O. Jolly, F. Jacqueline, and P. Claquin. 2022. Colimitation assessment of phytoplankton growth using a resource use efficiency approach in the Bay of Seine (French-English Channel). Journal of Environmental Management 306. https://doi.org/10.1016/j.jenvman.2022.114487.
Smayda, T.J., and V.L. Trainer. 2010. Dinoflagellate blooms in upwelling systems: Seeding, variability, and contrasts with diatom bloom behaviour. Progress in Oceanography 85: 92–107. https://doi.org/10.1016/j.pocean.2010.02.006.
Tilman, D. 1985. The resource-ratio hypothesis of plant succession. The American Naturalist 125: 827–852.
Utermöhl, H. 1958. Zur Vervollkommnung der quantitativen Phytoplankton-Methodik. Mitt. Internationale Ver. Theoretische Und Angewandte Limnologie 9: 1–38.
Venrick, E.L. 1978. How many cells to count? In Phytoplankton Manual, ed. A. Sournia, 167–180. Paris: UNESCO.
Vybernaite-Lubiene, I., M. Zilius, G. Giordani, J. Petkuviene, D. Vaiciute, P.A. Bukaveckas, and M. Bartoli. 2017. Effect of algal blooms on retention of N, Si and P in Europe’s largest coastal lagoon. Estuarine, Coastal and Shelf Science 194: 217–228. https://doi.org/10.1016/j.ecss.2017.06.020.
Wang, F., Y. Wei, G. Zhang, L. Zhang, and J. Sun. 2022. Picophytoplankton in the West Pacific Ocean: a snapshot. Frontiers in Microbiology 13. https://doi.org/10.3389/fmicb.2022.811227
Wasmund, N., J. Kownacka, J. Göbel, A. Jaanus, M. Johansen, I. Jurgensone, S. Lehtinen, and M. Powilleit. 2017. The diatom/dinoflagellate index as an indicator of ecosystem changes in the Baltic Sea 1. principle and handling instruction. Frontiers in Marine Science 4: 1–13. https://doi.org/10.3389/FMARS.2017.00022.
Weithoff, G., and B.E. Beisner. 2019. Measures and approaches in trait-based phytoplankton community ecology - from freshwater to marine ecosystems. Frontiers in Marine Science 6: 1–11. https://doi.org/10.3389/fmars.2019.00040.
Wulff, F., B.D. Eyre, and R. Johnstone. 2011. Nitrogen versus phosphorus limitation in a subtropical coastal embayment (Moreton Bay; Australia): Implications for management. Ecological Modelling 222: 120–130. https://doi.org/10.1016/j.ecolmodel.2010.08.040.
Yamamoto, T. 2003. The Seto Inland Sea - eutrophic or oligotrophic? Marine Pollution Bulletin 47: 37–42. https://doi.org/10.1016/S0025-326X(02)00416-2.
Zohary, T., B. Herut, M.D. Krom, R. Fauzi, C. Mantoura, P. Pitta, S. Psarra, F. Rassoulzadegan, et al. 2005. P-limited bacteria but N and P co-limited phytoplankton in the Eastern Mediterranean—a microcosm experiment. Deep Sea Research Part II: Topical Studies in Oceanography 52: 3011–3023. https://doi.org/10.1016/j.dsr2.2005.08.011.
Funding
Open access funding provided by FCT|FCCN (b-on). This work was financially supported by the Portuguese Foundation for Science and Technology (FCT) through projects UID/00350/2020 CIMA and LA/P/0069/2020 (Associate Laboratory ARNET). FCT also provided funding for R.B.D. through a postdoctoral fellowship and a researcher contract (SFRH/BPD/68688/2010, DL57/2016/CP1361/CT0017), and to P.N. through a Ph.D. fellowship (UI/BD/150772/2020).
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by Hans W. Paerl
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Domingues, R.B., Nogueira, P. & Barbosa, A.B. Co-Limitation of Phytoplankton by N and P in a Shallow Coastal Lagoon (Ria Formosa): Implications for Eutrophication Evaluation. Estuaries and Coasts 46, 1557–1572 (2023). https://doi.org/10.1007/s12237-023-01230-w
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12237-023-01230-w