Abstract
Biodiversity is important for communities to be resilient to a changing world, but patterns of diversity fluctuate naturally over time. Understanding these shifts — and the species driving community dynamics — is crucial for informing future ecological research and conservation management plans. We investigated the impacts of seasonality, small-scale changes in seagrass cover, and small-scale spatial location on the epifaunal communities occupying a temperate seagrass bed in the South Island of New Zealand. By sampling epifaunal communities using a fine-mesh push net two to three times per season for 1 year, and using a combination of multivariate and hierarchical diversity analyses, we discovered that season, seagrass cover, and the location within the bay, and their interactions, explained 88.5% of the variation in community composition. Community composition and abundances, but not numbers, of species changed over seasons. The most common taxa were commercially important Caridean shrimp and juvenile flounder (Rhombosolea spp.), and both decreased in abundance in summer (shrimp: 1.40/m2 in winter to 0.80/m2 in summer; flounder: 0.15/m2 in winter to 0.01/m2 in summer). Other commercially important species were captured as juveniles, including blue cod (Parapercis colias), kahawai (Arripis trutta), and whitebait (Galaxias spp.). The only adult fish captured in the study were two pipefish species (Stigmatopora nigra and Leptonotus elevatus), which had distinctly seasonal breeding patterns, with reproductively active adults most likely to be found in the spring and fall. Our study highlights the importance of estimating biodiversity parameters based on sampling throughout the year, as some species will be overlooked. We demonstrate that the temperate estuarine seagrass-affiliated animal communities differ in response to season and fine-scale local environments, causing fluctuations in biodiversity throughout the year.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Foundation species, such as seagrasses, mangroves, and coral reefs, provide ecosystem functions and services such as nutrient cycling, sediment trapping and stabilisation, attenuating water flow, and carbon sequestration (Costanza et al. 1997; Heck et al. 2003; Morrison et al. 2014; Nordlund et al. 2016; Ruiz-Frau et al. 2017; Orth et al. 2020). Moreover, these foundation species also facilitate biodiversity by providing habitat and supporting diverse food webs (Spalding et al. 2003; Gillanders 2006; Guannel et al. 2016). Uncovering the habitat components facilitating biodiversity is important for understanding the ecological impacts of predicted habitat loss (Waycott et al. 2009).
Estuaries with seagrass beds are known to harbour high biodiversity, in part because seagrass provides refugia to a large number of organisms (Orth and van Montfrans 1987; Orth et al. 2006; Grech et al. 2012), especially juvenile fish that rely on seagrass as a nursery habitat (Hemminga and Duarte 2000; Heck et al. 2003; Gillanders 2006; Shibuno et al. 2008; Bertelli and Unsworth 2014). Some species that use seagrass as a nursery include species important for fisheries industries, such as shrimp and prawns (Watson et al. 1993; Haywood et al. 1995; Schaffmeister et al. 2006; Taylor et al. 2017), flounder (Earl et al. 2014; Hyndes et al. 2018), and Australasian snapper (Schwarz et al. 2006). By providing shelter for prey species, seagrass has the ability to alter predator–prey relationships while simultaneously supporting species in other trophic levels such as sea turtles, dugongs, tiger sharks, and marine birds (Aragones and Marsh 1999; Beck et al. 2001; Heithaus et al. 2002; Turner and Schwarz 2006; Bertelli and Unsworth 2014). These nursery habitats might provide an influx of certain species during specific seasons, for example when juvenile fish settle out from larvae or when larger predators use nurseries as feeding grounds, thereby affecting seasonal patterns of community diversity.
Seagrass biomass can also fluctuate seasonally, impacting the species that rely on it as a habitat. Seagrass characteristics such as above-ground biomass, shoot density or height, and epiphyte biomass can positively correlate with the densities of certain species and overall community diversity (Orth et al. 1984; Ray et al. 2014). In temperate regions, seagrass traits such as density are impacted by seasonal changes in water temperature, light, day-length, salinity, nutrient concentrations, and dominant phytoplankton species (Hemminga and Duarte 2000; Jankowska et al. 2014). Temperate seagrass meadows often follow a cyclic pattern in response to these abiotic variables (Duarte 1989; Olesen and Sand-Jensen 1994), and faunal communities in seagrass beds likewise experience seasonal fluctuations (Bauer 1985; Edgar and Shaw 1995; Nakaoka et al. 2001; Ribeiro et al. 2006; Hutchinson et al. 2014; Jankowska et al. 2014; Włodarska-Kowalczuk et al. 2014). Fluctuations in seagrass and its associated communities also respond to more episodic or local stressors like algal blooms (Thomsen et al. 2012; Carstensen et al. 2015), marine heat waves (Oliver et al. 2018, 2019; Smale et al. 2019), and extreme weather events (Harris et al., 2020), which can be seasonally linked, but might also vary at fine spatial scales — for example, due to freshwater inputs carrying nutrients into coastal habitats; (Lapointe et al. 2015). These unpredictable environmental perturbations might counteract and complicate predictable cues in shaping estuarine biodiversity.
Furthermore, biodiversity of estuarine seagrass beds can also be impacted by seascape features such as fragmentation and loss of connectivity between biogenic habitats (Boström et al. 2011), for example created by freshwater inflows or large unvegetated areas (Boström et al. 2006), or by anthropogenic stressors like propeller scars, haul seining, and trampling (Orth et al. 2017; Uhrin and Holmquist 2003). For species that use seagrass as a nursery, proximity to habitats used as adults (e.g., rocky reefs) is an important predictor of species abundance (Rees et al. 2018). The spatial scale of the impacts of fragmentation between habitats differs based on the type of organism, and has been measured across a wide variety of scales (Boström et al. 2011). Biodiversity across interconnected seascapes, such as seagrass and mangrove habitats, can also fluctuate across seasons (Tarimo et al. 2022), as fauna transition between nursery grounds or change in response to biogenic habitat seasonality (Cuvillier et al. 2017).
Here, we quantify seasonal changes to epifaunal community diversity in a temperate seagrass bed in New Zealand. In this bioregion, only one species of seagrass occurs, Zostera muelleri (Turner and Schwarz 2006; Short et al. 2007), which has seasonal but rare sexual reproduction in New Zealand (Ramage and Schiel 1998; Dos Santos and Matheson 2017). Seagrass in New Zealand primarily occurs in intertidal mudflats of sheltered estuaries but has also been found on rocky reef platforms, coastal beaches, and in subtidal waters (Inglis 2003; Turner and Schwarz 2006; Morrison et al. 2014). Seagrass is declining throughout New Zealand due to anthropogenic impacts (Turner and Schwarz 2006; Matheson et al. 2011; Matheson and Wadhwa 2012; Morrison et al. 2014). In New Zealand, seagrass habitats have been suggested to be biodiversity hotspots, but seasonal data on epifaunal community structures supporting this notion remain sparse (Turner and Schwarz 2006; Morrison et al. 2014). More specifically, research on seasonal dynamics of epifaunal seagrass communities are lacking in terms of understanding small-scale habitat characteristics shaping biodiversity and have largely focussed on sampling in one season (e.g., van Houte-Howes et al. 2004; Morrison et al. 2014). Here, we address this research gap by testing for impacts of small-scale spatial variation, seagrass cover, and season on epifaunal community diversity in a New Zealand seagrass bed. We analyse population dynamics of three focal taxa for which we collected size or age data, providing a unique contribution to understanding the drivers of community diversity in an estuarine seagrass bed.
Methods
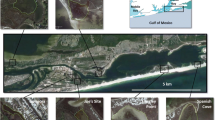
This study was conducted in Duvauchelle Bay (43.7504° S, 172.9334° E), located in the Akaroa Harbour on the South Island of New Zealand (Fig. 1). Duvauchelle Bay is one of the northernmost bays in Akaroa Harbour, over 1 km from the harbour mouth. The bay is approximately 800 m wide with mosaic patches of no seagrass (mud and sand flats) and sparse, medium (“patchy”), or consistent dense cover within close proximity to each other (Figs. 1 and S1). We assessed temporal variation in animal communities occupying the seagrass habitat in Duvauchelle Bay by sampling monthly from November 2019 through October 2020 (see Table S1 for detail on sampling dates and effort). We refer to the sampled animal communities as epifaunal communities, using a broad definition that encompasses invertebrates and fish found on or among the seagrass leaves (Chen et al. 2021). The full list of animals we include is listed in Table S2, as recommended by Chen et al. (2021).
Map of the sample location on the South Island of New Zealand (left) with the sampling locations within Duvauchelle Bay shown in the two images on the right. At the top right, sampling tows are shown colour-coded based on seasons (coral = spring, green = summer, yellow = fall, blue = winter). In the bottom right, tows are colour-coded based on seagrass cover (gradient of greens from light to dark representing bare to dense, for more details, see Fig. S1)
Monthly Sampling Methods
Each sampling event began approximately 2 h before low tide in the intertidal zone and sampled the shallow subtidal zone as the tide receded. To sample, we used a hand-hauled 1.2 m × 1.2-m push net (5-mm mesh) mounted on a PVC frame, dragging the net parallel to shore in a series of non-overlapping tows through 0.5–0.75-m-deep water. This method is ideal for capturing small animals with minimal impact on the seagrass beds (Thomsen et al. 2020). Because tows were a non-uniform length, we recorded time-stamped coordinates for the start and end of each tow using a Garmin GPS (average tow length was 30.6 m). At the end of each tow, the density of the seagrass was recorded based on a visual assessment from one of four semi-quantitative categories: bare patches, in which no seagrass was observed; sparse seagrass, in which some seagrass shoots were seen but the majority of the substrate was bare; patchy seagrass, when the tow included a mix of bare areas and seagrass, but more seagrass occurred than in sparse tows; or dense seagrass, when seagrass was the primary substrate visible (see Fig. S1 for representative drone images). Tows were conducted to remain within a single seagrass cover category as much as possible. All animals captured by the net in each tow were identified to the lowest taxonomic level (species for some, family or infraorder for others), and the counts of each type were recorded. Some taxa, including all fish, were recorded in size categories to capture ontogenetic variation throughout the year.
Pipefish species (Stigmatopora nigra and Leptonotus elevatus) were also identified by sex and classified as either adults or juveniles. Adult males of both species can be recognised by the presence of a brood pouch. We identified adult females based on body size and the presence of female ornamentation. All pipefish larger than 60 mm were tagged using visible implant fluorescent elastomer tags (Northwest Marine Technologies). Using established protocols for tagging syngnathid fishes for minimally invasive population monitoring (e.g., Masonjones et al. 2010; Flanagan et al. 2017; Masonjones and Rose 2019), pipefish were anesthetised using 100 mg/L of clove oil in 500 mL of seawater and three coloured tags in unique combinations (to facilitate individual identification) were injected just below the skin. Fish recovered in buckets of fresh seawater and were gently released back into the subtidal seagrass habitats. These methods were approved by the University of Canterbury Animal Ethics Committee (protocol # AEC2018/22R).
Analysis of Community Diversity
All analyses were conducted in R v. 4.1.0 (R Core Team 2021), and plots were created using packages magick (Ooms 2021), leaflet (Cheng et al. 2021), and mapview (Appelhans et al. 2021). Each sampling date was lumped by southern hemisphere season (Summer = Dec–Feb; Fall = Mar–May; Winter = June–Aug; Spring = Sep–Nov). We used the GPS coordinates from the start and stop of each tow to calculate the tow distances using the R package geosphere (Hijmans 2019) and multiplied the linear distances by the width of the net (1.2 m) to calculate the tow area. A single tow was removed from the analyses because the seagrass cover was not recorded.
To analyse effects associated with fine-scale spatial variation, we used distance-based Moran’s Eigenvector Mapping (dbMEM), a method which accounts for spatial autocorrelation in the analysis of community composition (Dray et al. 2006; Legendre and Legendre 2012). This approach is valuable because it accounts for interacting features of the bay impacting community composition such as seagrass cover, tidal zone, and unmeasured spatial features of the environment. This method uses eigenvalue mapping of the tow coordinates to reduce spatial data to a smaller number of axes that describe spatially autocorrelated community variation, which can then be used as predictor variables in a partial redundancy analysis of the community composition. The final model of relative densities of taxa included the effects of seagrass cover, season, the most informative axes describing small-scale spatial variation, and the overlap of each of those components. A full description of the analysis can be found in the Supplementary Methods. We used R packages adespatial (Dray et al. 2021), SoDA (Chambers 2020), vegan 2.5–7 (Oksanen et al. 2015).
A hierarchical analysis of patterns of diversity is most appropriate, given that our study design was hierarchical, with seagrass cover nested within sampling date, which itself is nested within season. The hierDiversity package (Marion et al. 2015a, b) is designed for such sampling designs, and estimates alpha, beta, and gamma diversity in a hierarchical framework for specified Hill numbers (Hill 1973; Jost 2007; Chao et al. 2014). Hill numbers are a unified set of diversity metrics, each associated with an order q, which down-weight rare species as q increases. When q = 0, all species carry equal weight (corresponding to traditional “species richness”); at q = 1, the weighted geometric mean is used, and the diversity measure is a transformed version of Shannon’s entropy; for q = 2, the weighted arithmetic mean of species is used, and abundant species have more influence on the diversity metric (Hill 1973; Jost 2007; Chao et al. 2014). Calculating multiple diversity estimates using multiple Hill numbers (i.e., creating “diversity profile plots”) allowed us to inspect the importance of dominant versus less abundant species in shaping diversity and turnover (Marion et al. 2015b, 2021). For this analysis, we calculated pairwise turnover (scripts available on GitHub: https://github.com/spflanagan/ecology-duvauchelle/tree/main/R/hierDiversity) to avoid biasing our beta diversity estimates due to uneven sampling across hierarchical levels (Marion et al. 2017). See Supplemental Methods for further details. Rarefaction analysis, done in vegan (Oksanen et al. 2015), indicated that some of our tows did not sufficiently capture the diversity of the community. Therefore, we created 999 rarefied communities, in which we down-sampled to the number of animals caught in the shortest tow, and ran our modified hierDiversity analysis on each of those communities. Using the distributions of these diversity estimates from the rarefied communities, we assessed whether the diversity profiles from our observed community were impacted by sampling effects. This analysis used the densities (the number of individuals divided by the tow area, to account for variation among tow areas) of all observations except those with zero animals captured in the tow.
To test for associations between particular taxonomic groups and seagrass cover and season, we used the multi-level pattern analysis in the R package indicspecies (Cáceres and Legendre 2009). We ran this analysis independently for seagrass cover and for season using the function multipatt to identify the “core” community membership associated with each test factor. The results of these analyses were compared to the species loadings from the distance-based Moran’s eigenvector maps analysis to identify species with strong associations with these two variables.
Population Dynamics of Focal Taxa
We further investigated the impacts of season and seagrass cover on the abundance of key taxa in Caridean shrimp, pātiki (flounder, Rhombosolea spp.), and pipefish (Stigmatopora nigra and Leptonotus elevatus). We focused on shrimp and pātiki because they were the two most abundant and frequently captured taxa in the dataset (see “Results”), and on pipefish because they were part of a larger study. Furthermore, we also measured sizes for these taxa, allowing us to test for seasonal ontogenetic changes. For each taxon, we fitted generalised linear models with the most appropriate link functions and used a model testing approach to identify the model with the lowest Akaike information criterion, corrected for small sample sizes (AICc).
Shrimp densities were normally distributed after log transformation and we therefore used a linear model (instead of a generalised linear model). The predictor variables included seagrass cover, season, and size class of the shrimp (≤ 50 mm or > 50 mm), and their interactions. Residual plots from the best-fitting model were evaluated for model fit and assumptions.
Flounder densities were not normally distributed after log-transformation nor were their residuals from linear models, and the raw counts were overdispersed (the mean counts, 1.59, were much smaller than the variance, 14.29). Data with these properties are best modelled using a zero-inflated negative binomial regression, here implemented in the pcsl package v. 1.5.5 (Zeileis et al. 2008; Jackman 2020). We modelled raw counts and included the tow area as a predictor. As with the shrimp data, we modelled counts predicted by seagrass cover, season, and size class (≤ 10 mm, 10–20 mm, 20–50 mm, > 50 mm). All four size classes are considered juveniles as we did not catch any fish > 15 cm (Earl et al. 2014). To assess the fit of the model, we visualised the residuals compared to each variable in the model to ensure residuals were centred around 0 and calculated the dispersion statistic as the \(\Sigma ({residuals}^{2})/(N-p-1)\), where N is the number of individuals and p is the number of parameters (including interaction coefficients). Furthermore, we created a confusion matrix of the 0 and non-zero observations to estimate the false negative rate. Finally, we used ordinary least squares regression to investigate the relationship between the observed non-zero counts and the non-zero counts predicted by the model. Specifically, we extracted the predicted counts and used bootstrapping implemented by the boot package v. 1.3–28 (Canty and Ripley 2020) to estimate confidence intervals.
Because pipefish were rare compared to flounder and shrimp (see results), and therefore their counts overdispersed and densities highly skewed, we modelled their presence, instead of abundance, using a multinomial logistic regression. This approach enabled us to predict the probability of encountering reproductively mature pipefish, given the season, seagrass cover, and pipefish species identity (Stigmatopora nigra vs Leptonotus elevatus). Photographs and size data were used to identify individuals as adults or juveniles and assign them to the appropriate species, and count data to match the individuals with the specific tows in which they were captured. Tows without pipefish were given the status “absent” instead of “juvenile” or “adult.” We then modelled the status of individual pipefish, predicted by seagrass cover, season, their interaction, and species identity using a multinomial log-linear regression implemented in nnet v. 7.3–16 (Ripley and Venables 2021). To ensure assumptions of the model were not violated, we visually inspected the residuals compared to each variable in the model. Finally, we used the effects package version 4.2–0 (Fox et al. 2020) to generate predicted probabilities and confidence intervals for each level of status across the predictor variables.
Results
A total of 403 tows covering 14,812 m2 were sampled over 12 dates, with a total of 133 tows in summer (3 dates), 52 in fall (2 dates), 108 in winter (3 dates), and 110 in spring (4 dates). We caught 24,511 animals, represented by 29 taxa, including 10 fish, 7 crabs, and five snails and whelks (see Table S2 for total counts and mean densities). The most abundant taxa were Caridean shrimp (67.72% of the total catch; all shrimp were identified to infraorder), followed by Rhombosolea spp. (10.65% of total catch) and Diloma subrostrata (5.75% of total catch). Species accumulation curves suggest that sampling effort was insufficient for most species except Rhombosolea spp. and Caridean shrimp (Fig. S2).
Community Composition and Diversity
Partitioning the variation in the seagrass communities revealed that the spatial position of the tows, seagrass cover, season, and their overlapping effects shaped the abundances of the animals in the seagrass beds, with each factor having a significant effect in partial redundancy analyses (P ≤ 0.001; Table S3). The distance-based redundancy analysis with the interacting effects of all three types of explanatory variables (seagrass cover, season, and space) was significant (F317,85 = 2.063, p = 0.001). The constrained axes explained 88.5% of the variation in community composition, with the first two axes accounting for ≥ 65% of variation (64.51% of total and 72.91% of constrained; Fig. 2A and B). Three of the 25 retained axes describing the spatial location of the tows had substantial loadings in the redundancy analysis, and those axes primarily corresponded to specific regions of the bay, matching bay-wide east–west and diagonal intertidal off-shore gradients (Fig. S3; Supplementary Results). Summer samples were most differentiated from other seasons (Fig. 2A) and were most similar to the ellipse describing samples from dense seagrass habitats (Fig. 2A and B). The species with the largest magnitude loadings on the first axis were Caridean shrimp, Rhombosolea spp., Austrovenus stutchburyi, and Potamopyrgus antipodarum, and on the second axis Diloma subrostrata, A. stutchburyi, P. antipodarum, Rhombosolea spp., and Caridean shrimp (Fig. 2B). Flounder were associated with variation in a direction orthogonal to dense habitats (i.e., they were least associated with dense seagrass), although the four seagrass cover classes had substantial overlap in the first two multivariate axes (Fig. 2B).
The first two constrained coordination analysis axes incorporating the effects of season, seagrass cover, and spatial covariates from a distance-based Moran’s eigenvalue matrix. A and B show the same axes (explaining 65% of the constrained variation) and sample points, but where overlain ordiellipses representing seasonal patterns (A) and seagrass cover (B), respectively. Also plotted are eigenvectors, scaled by eigenvalue, for the five taxa with the highest loadings
The alpha diversity profile plots suggest that, across hierarchical levels, community composition was dominated by a few abundant species, with most species occurring in low numbers. Both the observed (Fig. 3, top row) and the rarefied (Fig. 3, bottom row) communities showed that alpha diversity decreased with increasing q (i.e., as rare-species are down-weighted), with values for richness (q = 0) around 5 effective taxa per tow for each seagrass cover category and 20 for each season, both decreasing to 2 to 5 effective taxa per tow for all categories at q = 2 (the inverse of Simpson’s index). These patterns suggest that the communities are dominated by several highly abundant species, with most other species occurring at low abundances. However, we found no major differences in alpha diversity across seagrass cover or seasons, although estimates from the rarefied communities suggest that more species were present in the spring than in the other seasons (Fig. 3).
Diversity profile plots of alpha diversity for Hill’s q = 0, q = 1, and q = 2, calculated at the hierarchical levels of seagrass (A, D), nested within season (B, E), nested in the overall alpha diversity (C, F). These values reflect the effective number of taxa captured per tow. At q = 0, all species have the same weighting, but as q increases, rare species have less influence on the metric. The negative slopes on the profile plots indicate that common species drive patterns of species richness. A–C Observed alpha diversity values with bootstrap-estimated confidence intervals, D–F Estimates of alpha diversity and associated confidence intervals estimated from 999 permutation communities rarefied down to the smallest tow distance in the dataset (note that some confidence thresholds are so narrow that they are not visible in the plots)
The pairwise turnover plots suggest that within each level of seagrass cover, there were few disparities between common versus rare species (see flat profile plots in Fig. 4), suggesting that samples share dominant and low-abundance species within each seagrass habitat. These analyses suggest that sparse habitats had lower estimates of pairwise turnover than other seagrass types. However, at higher hierarchical levels (season and overall), communities differed in less abundant species but shared the same highly abundant species, as can be inferred by the diversity profile plots’ negative slopes (Marion et al. 2021). Again, seasons did not differ in the observed estimates, although the rarefied estimates suggested small differences, with larger disparity in communities between tows in fall compared to other seasons (Fig. 4).
Diversity profile plots of pairwise turnover for Hill’s q = 0, q = 1, and q = 2, calculated at the hierarchical levels of seagrass (A, D), nested within season (B, E), nested in the overall pairwise turnover (C, F). At q = 0, all species have the same weighting, but as q increases, rare species have less influence on the metric. The negative slopes on the profile plots indicate that common species drive patterns of sample dissimilarities. A–C Observed pairwise turnover values with bootstrap-estimated confidence intervals. D–F Estimates of pairwise turnover and associated confidence intervals estimated from 999 permutation communities rarefied down to the smallest tow distance in the dataset (note that some confidence thresholds are so narrow that they are not visible in the plots)
Abundances of individual seagrass-associated taxa followed the community-level analyses, in that only few species showed strong associations with individual variables (see overlapping ellipses in Fig. 2 and the generally overlapping diversity results). The most common species did not have strong associations with particular seagrass habitats in Duvauchelle Bay (Table S4), likely because they were found in reasonably large numbers in all habitat types (e.g., we caught 246 flounder in dense seagrass, but these represented only 9.58% of all flounder captured). Still, four taxa were associated with specific habitat types: Arripis trutta (indicator value = 0.342, p = 0.005), Polychaeta spp. (indicator value = 0.301, p = 0.005), and Notolabrus celidotus (indicator value = 0.292, p = 0.005) were associated with dense seagrass, whereas Retropinna retropinna were associated with patchy seagrass (indicator value = 0.232, p = 0.01; Table S4). Although no species were associated with bare habitats, four taxa were associated with seagrass presence (sparse, patchy, and dense habitats): Halicarcinus spp. (indicator value = 0.722, p = 0.005), blennies (indicator value = 0.687, p = 0.005), Stigmatopora nigra (indicator value = 0.405, p = 0.005), and Cominella glandiformis (indicator value = 0.321, p = 0.025). In addition, Peridotea ungulata (indicator value = 0.510, p = 0.005), Labridae (indicator value = 0.408, p = 0.005), and Leptonotus elevatus (indicator value = 0.254, p = 0.025; Table S4) were associated with patchy and dense seagrass habitats combined.
The tests of abundance patterns across seasons revealed that most taxa were associated with combinations of non-summer seasons (concordant with the multivariate analysis, Fig. 2). Specifically, seven taxa were associated with the winter-spring-fall combination, including Rhombosolea spp. (indicator value = 0.878, p = 0.005), Caridea (indicator value = 0.845, p = 0.005), Varunidae (indicator value = 0.567, p = 0.005), Peridotea ungulata (indicator value = 0.480, p = 0.005), Macrophthalmus hirtipes (indicator value = 0.467, p = 0.005), S. nigra (indicator value = 0.378, p = 0.015), and juvenile N. celidotus (indicator value = 0.262, p = 0.03). Juvenile R. retropinna and P. colias were associated with winter (Table S4), limpets (gastropods in subclass Patellogastropoda) with spring (indicator value = 0.204, p = 0.015), and juvenile A. trutta with summer (indicator value = 0288, p = 0.005). Furthermore, C. glandiformis was associated with the winter-spring combination (indicator value = 0.372, p = 0.005), juvenile Labridae with winter-fall (indicator value = 0.382, p = 0.005), Pyura pachydermatina with spring–summer (indicator value = 0.274, p = 0.02), and L. elevatus with spring-fall (indicator value = 0.255, p = 0.03; Table S4).
Population Dynamics of Focal Taxa
Caridean shrimp densities were best modelled with all three explanatory variables (seagrass cover, season, and individual size) and their interactions. Although the model was statistically significant (F31,774 = 10.73, p < 0.001), it explained only 27.25% of the variation in the data. Patchy seagrass and sparse seagrass had significant positive effects on shrimp densities (Table S5; patchy: estimate = 0.511 ± 0.168, t = 3.039, p = 0.00245; sparse: estimate = 0.705 ± 0.180, t = 3.912, p < 0.001). However, interactions contributed negatively to shrimp densities (i.e., decreases in shrimp densities were explained by combinations of particular seasons and habitat types), in particular the interactions of summer × patchy (estimate = −0.638 ± 0.206, t = −3.098, p = 0.002), spring × sparse (estimate = −0.541 ± 0.223, t = −2.43, p = 0.015), summer × sparse (estimate = −0.979 ± 0.218, t = −4.488, p < 0.001), and spring × large size (estimate = −0.450 ± 0.203, t = −2.212, p = 0.027).
The best-fitting zero-inflated negative binomial model of flounder (Rhombosolea spp.) abundances included an interaction between season and seagrass cover, with additive effects of size and tow area (see Supplementary Results for details of model fit). Flounder showed a strong negative decline in summer (β = −2.00 ± 0.477, z = −4.199, p < 0.001) and were most abundant in bare habitats (β = 1.705 ± 0.182, z = 9.381, p < 0.001; Fig. 6). The two larger size classes were less abundant than the smallest sizes (20–50 mm: β = −0.532 ± 0.144, z = −3.687, p < 0.001; > 50 mm: β = −2.252 ± 0.413, z = −5.457, p < 0.001). Of the interactions, the only significant effects were sparse seagrass × summer (β = 1.437 ± 0.559, z = 2.572, p = 0.010) and patchy seagrass × summer (β = 1.871 ± 0.616, z = 3.036, p = 0.002). In addition, the log(θ) term was significant (β = 0.341 ± 0.132, z = 2.586, p = 0.010), confirming our assessment that the count data were overdispersed. Similar patterns were seen for the zero-inflation component of the model (see Table S6 for all coefficients).
The two pipefish species had largely similar responses to seagrass cover, season, and their interaction (Table S7). Both species were found in low abundances, and our model reflects this by predicting the probability of absences in a given tow, in any habitat or season, as > 0.8. Pipefish were least likely to be observed in bare habitats, regardless of other variables, and most likely to be observed in patchy or dense seagrass habitats (Fig. 7). Both species of all ages had the lowest predicted probabilities in those preferred habitats during the summer (predicted probabilities of being captured in a given tow < 0.05) and the highest probabilities in the spring and fall for both age categories (Fig. 7). In the winter, the two age classes differed, with adults having near-zero capture probabilities but juveniles showing no substantial decline from fall to spring (Fig. 7). None of the 81 marked individuals were recaptured, so we are unable to estimate population sizes based on recapture rates.
Discussion
We found that seagrass cover, season, and small-scale spatial location explain community diversity and structure in Duvauchelle Bay, New Zealand. The communities were defined by the most abundant species (in particular, shrimp and flounder), and across seasons, the communities differed in their least abundant species but not the highly abundant species. Despite the importance of seasonality in shaping the community composition (i.e., which rare species were present), seasons only had minor effects on universal community diversity metrics. Our combination of multivariate statistical analyses and hierarchical diversity partitioning allowed us to maximise the insights gained from monthly sampling data.
Fine-scale spatial factors shaped community composition in Duvauchelle Bay. The three spatial axes substantially contributing to multivariate community diversity axes were associated with four distinct regions of the bay that would not have been easily described by other categorical variables such as intertidal region or the side of the bay (Fig. S3). Instead, these axes appear to correspond to fine-scale seascape features related to specific locations in the bay (Fig. S3). We hypothesise that these fine-scale features are related to a natural break in connectivity between the east and west sides of the bay caused by a freshwater inflow, varying high tide depths and exposures at low tide, and proximity to other habitat types including a rocky mussel reef on the edges of the bay. In this way, our data-driven approach enabled us to identify and include in our analysis seascape features that would have otherwise been overlooked. Further research is needed to conduct fine-scale mapping of these seascape features in Duvauchelle Bay and throughout Akaroa Harbour to enable incorporating these specific features into the analysis of community diversity (as done in Henderson et al. 2017).
While the mechanisms contributing to the importance of these spatial variables are unknown, fine-scale effects of seagrass traits have been shown to impact predation rates on small fishes (Chacin and Stallings 2016) and fish community diversity (Henderson et al. 2017), and factors such as wave exposure can impact fish assemblages (Perry et al. 2017). In line with our results emphasising the interacting effects of fine spatial scale variation and season, shallow-water fish communities in Zanzibar were impacted by offshore variables (e.g., wave exposure) in different ways across the seasons (Perry et al. 2017). Our study was not designed to detect broad-scale seascape features, nor were we able to incorporate seagrass traits in our analyses, but these are fruitful areas of future study as fish diversity has been shown to respond to both fine- and broad-scale habitat features (Berkström et al. 2013) and proximity to seascape features such as the open ocean (Henderson et al. 2017). Furthermore, the connectivity to the nearby habitats in other bays (including seagrass meadows, rocky reefs, and kelp forests) is likely to be an important factor impacting the biodiversity we report here (Cuvillier et al. 2017; Henderson et al. 2017; Rees et al. 2018; Tarimo et al. 2022).
The effect of seasons on the animal communities was — in part — driven by declines in the abundance of the most common animals (flounder and shrimp) in the summer. These declines were counteracted by increases in abundance of rare species, as reflected in the alpha diversity profile plots (summer having the largest alpha diversity at q = 1 and q = 2). This summer decline was surprising, in part because these taxa have previously been shown to peak in summer in other locations (Webb 1972; Bauer 1985), and also because fish are expected to be most common in New Zealand seagrasses in summer (Morrison et al. 2014). We suggest that the observed decreases can be attributed to macroalgal blooms observed in late January. Macroalgal blooms typically have negative impacts on seagrasses (Höffle et al. 2012), particularly in summer months, as well as on invertebrate communities in sedimentary habitats (Lyons et al. 2012). However, in lower abundances, macroalgae can also provide complex habitats and refugia for some species (Lyons et al. 2012; Thomsen and Wernberg 2015), which could explain the moderate increase in alpha diversity in the summer months. Another possibility is that the macroalgae reduced our ability to capture different species, in particular the more mobile flounder and shrimp.
Our result that less abundant species had highest turnover across seasons reflects patterns in fish recruitment. Most juvenile fish were captured only during one season (Table S1), which likely reflects species-specific variation in spawning habits. For example, smelt and blue cod juveniles were only found in winter, and kahawai mainly in summer. These timings correspond to breeding seasons as blue cod breed in winter and spring in Otago (Robertson 1973) and kahawai in Canterbury breed in summer (Webb 1973). Only typical seagrass residents such as pipefish, blenny, and small wrasses (Kendrick and Hyndes 2003; Gillanders 2006) and juvenile flounder were found year-round. All flounder captured in this study were small young-of-the-year (all < 100 mm, which is less than 2-year-old flounder (Webb 1972)), suggesting that Duvauchelle Bay serves as an important nursery ground, especially given the abundance of food (e.g., shrimp) and habitat complexity. However, whether this pattern can be directly attributed to the seagrass is uncertain, because juvenile flounder did not show a strong preference for patchy or dense seagrass and are often also found on mudflats (Webb 1972; Earl et al. 2014; Thomsen et al. 2020). Additionally, our sampling method targets small fishes across small spatial scales, so the occupancy of the seagrass by adults is unknown. Experimental studies are needed to identify the underpinning factors that cause seagrass beds in New Zealand to be valuable nursery habitats for juvenile fish. Understanding these factors is also of cultural and economic importance, as flounder are taonga (a treasure) to the local hapū (sub-tribe) as mahinga kai (traditional food source).
The interaction of seagrass cover and season was an important predictor in every model we analysed, likely because temperate seagrass cover typically fluctuates throughout the year (e.g., Jankowska et al. 2014). As in other systems (Włodarska-Kowalczuk et al. 2014), the magnitude of the differences along our bare-to-dense gradient of seagrass cover changed throughout the season for our focal species (Figs. 5, 6, and 7). Although spatial variability in seagrass density at small scales has been demonstrated to be important for diversity of epifaunal invertebrates (Gullström et al. 2012), the seasonal impacts of this small-scale variability are unclear. Furthermore, seagrass biomass and community diversity could have been impacted by (unmeasured) fine-scale features such as a freshwater input in the middle of the bay (e.g., Lirman and Cropper 2003) or nearby bivalve beds (e.g., Sharma et al. 2016), as seascape features are known to be important factors shaping faunal diversity (Boström et al. 2006; Cuvillier et al. 2017; Perry et al. 2017; Rees et al. 2018; Tarimo et al. 2022).
Mean shrimp abundances (± standard errors) were determined by season, seagrass cover, and their size. Two size categories are represented by different colours and shapes, and in all seasons except fall, smaller shrimp were more abundant. A–D show the predicted densities (ln(density + 1)) and E–H show the observed number of shrimp in bare (A, E), sparse (B, F), patchy (C, G), and dense (D, H) seagrass covers
Mean flounder abundances (± standard errors) were determined by habitat type (A, E bare, B, F sparse, C, G patchy, D, H dense seagrass), flounder size (represented by shapes and colours), and season. Flounder were most commonly found in bare habitats as very small juveniles, and were least commonly observed in the summer. The predicted number of flounder caught per tow (density, counts/m2) from the zero-inflated negative binomial model are presented with bootstrapped confidence intervals in A and D. The mean observed counts are shown with standard errors in E and H
Predicted probabilities (± standard errors) of each species being present as adults (A, C), or present as juveniles (B, C) in each season and across each level of seagrass cover (represented by different shapes and colours). Note the different y-axes. Adult pipefish were most likely to occur in spring and fall, whereas juveniles had similar likelihoods of occurring year-round in both species. Stigmatopora nigra juveniles were more likely to be caught than Leptonotus elevatus juveniles in every season. Both species were most likely to occur in dense or patchy seagrass habitats
We captured breeding adult pipefish, which are considered flagship seagrass species (Shokri et al. 2009) and indicators of healthy seagrass meadows (Shokri et al. 2009; Ford et al. 2010; Scapin et al. 2018). Breeding adults were absent in the winter, but juveniles remained likely to be captured (Fig. 7). These patterns suggest that the local pipefish population could be annual with adult mortality at the end of the summer, a hypothesis supported by a short life span observed for S. nigra in Australia (Parkinson et al. 2012; Parkinson and Booth 2016). Alternatively, the local pipefish populations might not be obligate seagrass dwellers, in contrast to consensus data (Hyndes et al. 2018), and adults could migrate to deeper waters and other habitats (e.g., kelp beds) upon completion of breeding. However, this migration hypothesis deviates from patterns observed in S. nigra in Australian seagrass beds (Connolly 1994; Hindell et al. 2000; Burt 2002; Browne et al. 2008; Smith et al. 2011). Regardless, we observed a shortened breeding season compared to the Australian populations, where breeding adults were found throughout the winter (Parkinson and Booth 2016), potentially supporting the hypothesis that populations of S. nigra in New Zealand and Australia have diverged sufficiently to be considered different species (Dawson 2012). A shortened breeding season could also contribute to stronger sexual selection (Emlen and Oring 1977) and thereby increase genetic divergence.
In conclusion, we have shown that the animal communities inhabiting a temperate seagrass bed varied across seagrass patches and seasons, with rarer species showing high temporal turnover. We also suggest that poorly studied seagrass beds on the South Island of New Zealand function as nursery grounds for flounder and habitat for iconic pipefish. These results highlight the importance of sampling many times over a year to capture all communities. Although the most abundant species (shrimp and flounder) dominated all habitat types, seagrass cover was an important factor facilitating several less common species, in particular pipefishes of all ages. Seagrass beds therefore enhance the biodiversity of the system. Seagrass abundance is declining globally (Orth et al. 2006) and in New Zealand (Turner and Schwarz 2006; Tan et al. 2020), and protecting this habitat is critical for maintaining biodiversity. Our finding that seasonality interacts with seagrass cover to shape community diversity further highlights the importance of conserving this habitat.
Data Accessibility
All code to reproduce the analyses is available on github (https://github.com/spflanagan/ecology-duvauchelle) and archived on zenodo (https://zenodo.org/account/settings/github/repository/spflanagan/ecology-duvauchelle). Data are available at https://zenodo.org/record/6800784.
References
Appelhans, T., F. Detsch, C. Reudenbach, S. Woellauer, S. Forteva, T. Nauss, E. Pebesma, et al. 2021. mapview: interactive viewing of spatial data in R (version 2.10.0).
Aragones, L., and H. Marsh. 1999. Impact of Dugong grazing and turtle cropping on tropical seagrass communities. Pacific Conservation Biology 5. CSIRO PUBLISHING: 277–288. https://doi.org/10.1071/pc000277.
Bauer, R.T. 1985. Diel and seasonal variation in species composition and abundance of caridean shrimps (Crustacea, Decapoda) from seagrass meadows on the north coast of Puerto Rico. Bulletin of Marine Science 36: 150–162.
Beck, M.W., K.L. Heck, K.W. Able, D.L. Childers, D.B. Eggleston, B.M. Gillanders, B. Halpern, et al. 2001. The identification, conservation, and management of estuarine and marine nurseries for fish and invertebrates: A better understanding of the habitats that serve as nurseries for marine species and the factors that create site-specific variability in nursery quality will improve conservation and management of these areas. BioScience 51: 633–641. https://doi.org/10.1641/0006-3568(2001)051[0633:TICAMO]2.0.CO;2.
Berkström, C., R. Lindborg, M. Thyresson, and M. Gullström. 2013. Assessing connectivity in a tropical embayment: Fish migrations and seascape ecology. Biological Conservation 166: 43–53.
Bertelli, C.M., and R.K.F. Unsworth. 2014. Protecting the hand that feeds us: seagrass (Zostera marina) serves as commercial juvenile fish habitat. Marine Pollution Bulletin 83. Seagrass Meadows in a Globally Changing Environment: 425–429. https://doi.org/10.1016/j.marpolbul.2013.08.011.
Boström, C., E.L. Jackson, and C.A. Simenstad. 2006. Seagrass landscapes and their effects on associated fauna: A review. Estuarine, Coastal and Shelf Science 68: 383–403.
Boström, C., S.J. Pittman, C. Simenstad, and R.T. Kneib. 2011. Seascape ecology of coastal biogenic habitats: Advances, gaps, and challenges. Marine Ecology Progress Series 427: 191–217.
Browne, R., J. Baker, and R. Connolly. 2008. Syngnathids: Seadragons, Seahorses, and pipefishes of Gulf St Vincent. In Natural History of Gulf St Vincent, 162–176. Royal Society of South Australia (Inc).
Burt, M. 2002. Does plant morphology influence fish fauna associated with seagrass meadows? Theses : Honours.
Cáceres, M.D., and P. Legendre. 2009. Associations between species and groups of sites: Indices and statistical inference. Ecology 90: 3566–3574. https://doi.org/10.1890/08-1823.1.
Canty, A., and B.D. Ripley. 2020. boot: Bootstrap R (S-Plus) Functions (version R package version 1.3–25).
Carstensen, J., R. Klais, and J. E. Cloern. 2015. Phytoplankton blooms in estuarine and coastal waters: seasonal patterns and key species. Estuarine, Coastal and Shelf Science 162. Special Issue: Global Patterns of Phytoplankton Dynamics in Coastal Ecosystems: 98–109. https://doi.org/10.1016/j.ecss.2015.05.005.
Chacin, D.H., and C.D. Stallings. 2016. Disentangling fine- and broad-scale effects of habitat on predator-prey interactions. Journal of Experimental Marine Biology and Ecology 483: 10–19.
Chambers, J.M. 2020. SoDA: Functions and Examples for “Software for Data Analysis” (version 1.0–6.1).
Chao, A., C.-H. Chiu, and L. Jost. 2014. unifying species diversity, phylogenetic diversity, functional diversity, and related similarity and differentiation measures through hill numbers. Annual Review of Ecology, Evolution, and Systematics 45: 297–324. https://doi.org/10.1146/annurev-ecolsys-120213-091540.
Chen, Y.-Y., G.J. Edgar, and R.J. Fox. 2021. The nature and ecological significance of epifaunal communities within marine ecosystems. In Oceanography and Marine Biology: CRC Press.
Cheng, J., B. Karambelkar, Y. Xie, H. Wickham, K. Russell, K. Johnson, B. Schloerke, et al. 2021. leaflet: Create Interactive Web Maps with the JavaScript “Leaflet” Library (version 2.0.4.1).
Connolly, R.M. 1994. A comparison of fish assemblages from seagrass and unvegetated areas of a southern Australian estuary. Marine and Freshwater Research 45: 1033–1044. https://doi.org/10.1071/mf9941033.
Costanza, R., R. d’Arge, R. de Groot, S. Farber, M. Grasso, B. Hannon, K. Limburg, et al. 1997. The value of the world’s ecosystem services and natural capital. Nature 387: 253–260. https://doi.org/10.1038/387253a0.
Cuvillier, A., N. Villeneuve, E. Cordier, J. Kolasinski, L. Maurel, N. Farnier, and P. Frouin. 2017. Causes of seasonal and decadal variability in a tropical seagrass seascape (Reunion Island, south western Indian Ocean). Estuarine, Coastal and Shelf Science 184: 90–101.
Dawson, M.N. 2012. Parallel phylogeographic structure in ecologically similar sympatric sister taxa. Molecular Ecology 21: 987–1004. https://doi.org/10.1111/j.1365-294X.2011.05417.x.
Dos Santos, V.M., and F.E. Matheson. 2017. Higher seagrass cover and biomass increases sexual reproductive effort: A rare case study of Zostera muelleri in New Zealand. Aquatic Botany 138: 29–36. https://doi.org/10.1016/j.aquabot.2016.12.003.
Dray, S., D. Bauman, G. Blanchet, D. Borcard, S. Clappe, G. Guenard, T. Jombart, et al. 2021. adespatial: multivariate multiscale spatial analysis (version 0.3–14).
Dray, S., P. Legendre, and P.R. Peres-Neto. 2006. Spatial modelling: A comprehensive framework for principal coordinate analysis of neighbour matrices (PCNM). Ecological Modelling 196: 483–493. https://doi.org/10.1016/j.ecolmodel.2006.02.015.
Duarte, C. 1989. Temporal biomass variability and production/biomass relationships of seagrass communities. Marine Ecology Progress Series 51: 269–276. https://doi.org/10.3354/meps051269.
Earl, J., A.J. Fowler, Q. Ye, and S. Dittmann. 2014. Age validation, growth and population characteristics of greenback flounder (Rhombosolea tapirina) in a large temperate estuary. New Zealand Journal of Marine and Freshwater Research 48. Taylor & Francis: 229–244. https://doi.org/10.1080/00288330.2013.875928.
Edgar, G.J., and C. Shaw. 1995. The production and trophic ecology of shallow-water fish assemblages in southern Australia I. Species richness, size-structure and production of fishes in Western Port, Victoria. Journal of Experimental Marine Biology and Ecology 194: 53–81. https://doi.org/10.1016/0022-0981(95)00083-6.
Emlen, S.T., and L.W. Oring. 1977. Ecology, sexual selection, and the evolution of mating systems. Science 197: 215–223.
Flanagan, S.P., G. Rosenqvist, and A.G. Jones. 2017. Mate quality and the temporal dynamics of breeding in a sex-role-reversed pipefish, S. typhle. Behavioral Ecology and Sociobiology 71. https://doi.org/10.1007/s00265-016-2255-3.
Ford, J., R. Williams, A. Fowler, D. Cox, and I. Suthers. 2010. Identifying critical estuarine seagrass habitat for settlement of coastally spawned fish. Marine Ecology Progress Series 408: 181–193. https://doi.org/10.3354/meps08582.
Fox, J., S. Weisberg, B. Price, M. Friendly, J. Hong, R. Andersen, D. Firth, S. Taylor, and R Core Team. 2020. Effect displays for linear, generalized linear, and other models. R (version 4.2–0).
Gillanders, B.M. 2006. Seagrasses, Fish, and Fisheries. In SEAGRASSES: BIOLOGY, ECOLOGYAND CONSERVATION, ed. A. W. D. LARKUM, R. J. ORTH, and C. M. DUARTE, 503–505. Dordrecht: Springer Netherlands. https://doi.org/10.1007/978-1-4020-2983-7_21.
Grech, A., K. Chartrand, P. Erftemeijer, M. Fonseca, L. McKenzie, M. Rasheed, H. Taylor, and R. Coles. 2012. A comparison of threats, vulnerabilities and management approaches in global seagrass bioregions. Environmental Research Letters 7. https://doi.org/10.1088/1748-9326/7/2/024006.
Guannel, G., K. Arkema, P. Ruggiero, and G. Verutes. 2016. The power of three: coral reefs, seagrasses and mangroves protect coastal regions and increase their resilience. PLOS ONE 11. Public Library of Science: e0158094. https://doi.org/10.1371/journal.pone.0158094.
Gullström, M., S. Baden, and M. Lindegarth. 2012. Spatial patterns and environmental correlates in leaf-associated epifaunal assemblages of temperate seagrass (Zostera marina) meadows. Marine Biology 159: 413–425. https://doi.org/10.1007/s00227-011-1819-z.
Harris, R.M.B., F. Loeffler, A. Rumm, C. Fischer, P. Horchler, M. Scholz, F. Foeckler, and K. Henle. 2020. Biological responses to extreme weather events are detectable but difficult to formally attribute to anthropogenic climate change. Scientific Reports 10: 14067. https://doi.org/10.1038/s41598-020-70901-6.
Haywood, M.D.E., D.J. Vance, and N.R. Loneragan. 1995. Seagrass and algal beds as nursery habitats for tiger prawns (Penaeus semisulcatus and P. esculentus) in a tropical Australian estuary. Marine Biology 122: 213–223.
Heck, K.L., G. Hays, and R.J. Orth. 2003. Critical evaluation of the nursery role hypothesis for seagrass meadows. Marine Ecology Progress Series 253: 123–136. https://doi.org/10.3354/meps253123.
Heithaus, M., L. Dill, G. Marshall, and B. Buhleier. 2002. Habitat use and foraging behavior of tiger sharks (Galeocerdo cuvier) in a seagrass ecosystem. Marine Biology 140: 237–248. https://doi.org/10.1007/s00227-001-0711-7.
Hemminga, M.A., and C.M. Duarte. 2000. Seagrass ecology. Cambridge University Press.
Henderson, C.J., B.L. Gilby, S.Y. Lee, and T. Stevens. 2017. Contrasting effects of habitat complexity and connectivity on biodiversity in seagrass meadows. Marine Biology. 164: 117.
Hijmans, R.J. 2019. geosphere: spherical trigonometry (version 1.5–10).
Hill, M.O. 1973. Diversity and evenness: A unifying notation and its consequences. Ecology 54: 427–432. https://doi.org/10.2307/1934352.
Hindell, J.S., G.P. Jenkins, and M.J. Keough. 2000. Variability in abundances of fishes associated with seagrass habitats in relation to diets of predatory fishes. Marine Biology 136: 725–737. https://doi.org/10.1007/s002270050732.
Höffle, H., T. Wernberg, M.S. Thomsen, and M. Holmer. 2012. Drift algae, an invasive snail and elevated temperature reduce ecological performance of a warm-temperate seagrass, through additive effects. Marine Ecology Progress Series 450: 67–80. https://doi.org/10.3354/meps09552.
Hutchinson, N., G.P. Jenkins, A. Brown, and T.M. Smith. 2014. Variation with depth in temperate seagrass-associated fish assemblages in Southern Victoria, Australia. Estuaries and Coasts 37: 801–814. https://doi.org/10.1007/s12237-013-9742-9.
Hyndes, G.A., P. Francour, P. Guidetti, and K.L. Heck. 2018. The roles of seagrasses in structuring associated fish assemblages and fisheries. In Seagrasses of Australia: Structure, Ecology and Conservation, ed. A. W. D. Larkum, G. A. Kendrick, and P. J. Ralph, 589–627. Cham: Springer International Publishing. https://doi.org/10.1007/978-3-319-71354-0.
Inglis, G. 2003. The seagrasses of New Zealand. World atlas of seagrasses. Univ of California Press: 134.
Jackman, S. 2020. pscl: Classes and methods for R developed in the political science computational laboratory. Sydney: New South Wales, Australia.
Jankowska, E., M. Włodarska-Kowalczuk, L. Kotwicki, P. Balazy, and K. Kuliński. 2014. Seasonality in vegetation biometrics and its effects on sediment characteristics and meiofauna in Baltic seagrass meadows. Estuarine, Coastal and Shelf Science 139: 159–170. https://doi.org/10.1016/j.ecss.2014.01.003.
Jost, L. 2007. Partitioning diversity into independent alpha and beta components. Ecology 88: 2427–2439. https://doi.org/10.1890/06-1736.1.
Kendrick, A.J., and G.A. Hyndes. 2003. Patterns in the abundance and size-distribution of syngnathid fishes among habitats in a seagrass-dominated marine environment. Estuarine, Coastal and Shelf Science 57: 631–640. https://doi.org/10.1016/S0272-7714(02)00402-X.
Lapointe, B.E., L.W. Herren, D.D. Debortoli, and M.A. Vogel. 2015. Evidence of sewage-driven eutrophication and harmful algal blooms in Florida’s Indian River Lagoon. Harmful Algae 43: 82–102. https://doi.org/10.1016/j.hal.2015.01.004.
Legendre, P., and L. Legendre. 2012. Numerical Ecology. Elsevier.
Lirman, D., and W.P. Cropper. 2003. The influence of salinity on seagrass growth, survivorship, and distribution within Biscayne Bay, Florida: Field, experimental, and modeling studies. Estuaries 26: 131–141. https://doi.org/10.1007/BF02691700.
Lyons, D.A., R.C. Mant, F. Bulleri, J. Kotta, G. Rilov, and T.P. Crowe. 2012. What are the effects of macroalgal blooms on the structure and functioning of marine ecosystems? A systematic review protocol. Environmental Evidence 1: 7. https://doi.org/10.1186/2047-2382-1-7.
Marion, Z.H., J.A. Fordyce, and B. Fitzpatrick. 2015a. HierDiversity: hierarchical multiplicative partitioning of complex phenotypes (version 0.1).
Marion, Z.H., J.A. Fordyce, and B.M. Fitzpatrick. 2015b. Extending the concept of diversity partitioning to characterize phenotypic complexity. The American Naturalist 186: 348–361. https://doi.org/10.1086/682369.
Marion, Z.H., J. Fordyce, and B. Fitzpatrick. 2017. Pairwise beta diversity resolves an underappreciated source of confusion in calculating species turnover - Marion - 2017 - Ecology - Wiley Online Library. Ecology 98: 933–939.
Marion, Z.H., K.H. Orwin, J.R. Wood, R.J. Holdaway, and I.A. Dickie. 2021. Land use, but not distance, drives fungal beta diversity. Ecology n/a: e03487. https://doi.org/10.1002/ecy.3487.
Masonjones, H.D., and E. Rose. 2019. When more is not merrier: using wild population dynamics to understand the effect of density on ex situ seahorse mating behaviors. PLOS ONE 14. Public Library of Science: e0218069. https://doi.org/10.1371/journal.pone.0218069.
Masonjones, H.D., E. Rose, L.B. McRae, and D. Dixson. 2010. An examination of the population dynamics of syngnathid fishes within Tampa Bay, Florida, USA. Current Zoology 56: 118–133.
Matheson, F.E., C.J. Lundquist, C.E.C. Gemmill, and C.A. Pilditch. 2011. New Zealand seagrass – more threatened than IUCN review indicates. Biological Conservation 144: 2749–2750. https://doi.org/10.1016/j.biocon.2011.08.020.
Matheson, F.E., and S. Wadhwa. 2012. Seagrass in Porirua Harbour: preliminary assessment of restoration potential. NIWA Report HAM2012–037. Hamilton, New Zealand: National Institute of Water & Atmospheric Research Ltd.
Morrison, M., M.L. Lowe, C. Grant, P. Smith, G. Carbines, J. Reed, S. Bury, and J. Brown. 2014. Seagrass meadows as biodiversity and productivity hotspots. 137. New Zealand Aquatic Environment and Biodiversity Report. Wellington, New Zealand: Ministry for Primary Industries.
Nakaoka, M., T. Toyohara, and M. Matsumasa. 2001. Seasonal and between-substrate variation in mobile epifaunal community in a multispecific seagrass bed of Otsuchi Bay, Japan. Marine Ecology 22. John Wiley & Sons, Ltd: 379–395. https://doi.org/10.1046/j.1439-0485.2001.01726.x.
Nordlund, L.M., E.W. Koch, E.B. Barbier, and J.C. Creed. 2016. seagrass ecosystem services and their variability across genera and geographical regions. PLOS ONE 11. Public Library of Science: e0163091. https://doi.org/10.1371/journal.pone.0163091.
Oksanen, J., F.G. Blanchet, R. Kindt, P. Legendre, P.R. Minchin, R.B. O’Hara, G.L. Simpson, P. Solymos, M.H.H. Stevens, and H. Wagner. 2015. vegan: community ecology package.
Olesen, B., and K. Sand-Jensen. 1994. Biomass-density patterns in the temperate seagrass Zostera marina. Marine Ecology Progress Series 9: 283–291.
Oliver, E.C.J., M.T. Burrows, M.G. Donat, A. Sen Gupta, L.V. Alexander, S.E. Perkins-Kirkpatrick, J.A. Benthuysen, et al. 2019. Projected marine heatwaves in the 21st century and the potential for ecological impact. Frontiers in Marine Science 6: 734. https://doi.org/10.3389/fmars.2019.00734.
Oliver, E.C.J., V. Lago, A.J. Hobday, N.J. Holbrook, S.D. Ling, and C.N. Mundy. 2018. Marine heatwaves off eastern Tasmania: Trends, interannual variability, and predictability. Progress in Oceanography 161: 116–130. https://doi.org/10.1016/j.pocean.2018.02.007.
Ooms, J. 2021. magick: Advanced Graphics and Image-Processing in R (version 2.7.3).
Orth, R.J., T.J.B. Carruthers, W.C. Dennison, C.M. Duarte, J.W. Fourqurean, K.L. Heck, A.R. Hughes, et al. 2006. A global crisis for seagrass ecosystems. BioScience 56: 987–996. https://doi.org/10.1641/0006-3568(2006)56[987:agcfse]2.0.co;2.
Orth, R.J., K.L. Heck, and J. van Montfrans. 1984. Faunal communities in seagrass beds: A review of the influence of plant structure and prey characteristics on predator-prey relationships. Estuaries 7: 339–350.
Orth, R. J., J.S. Lefcheck, K.S. McGlathery, L. Aoki, M.W. Luckenbach, K.A. Moore, M. P.J. Oreska, R. Snyder, D.J. Wilcox, and B. Lusk. 2020. Restoration of seagrass habitat leads to rapid recovery of coastal ecosystem services. Science Advances 6: eabc6434. https://doi.org/10.1126/sciadv.abc6434.
Orth, R.J., J.S. Lefcheck, and D.J. Wilcox. 2017. Boat propeller scarring of seagrass beds in lower Chesapeake Bay, USA: Patterns, causes, recovery, and management. Estuaries and Coasts 40: 1666–1676.
Orth, R.J., and J. van Montfrans. 1987. Utilization of a seagrass meadow and tidal marsh creek by blue crabs Callinectes sapidus. I. Seasonal and annual variations in abundance with emphasis on post-settlement juveniles. Marine Ecology Progress Series 41. Inter-Research Science Center: 283–294.
Parkinson, K.L., and D.J. Booth. 2016. Rapid growth and short life spans characterize pipefish populations in vulnerable seagrass beds. Journal of Fish Biology 88: 1847–1855. https://doi.org/10.1111/jfb.12950.
Parkinson, K.L., D.J. Booth, and J.E. Lee. 2012. Validation of otolith daily increment formation for two temperate syngnathid fishes: The pipefishes Stigmatopora argus and Stigmatopora nigra. Journal of Fish Biology 80: 698–704. https://doi.org/10.1111/j.1095-8649.2011.03194.x.
Perry, D., T.A.B. Staveley, L. Hammar, A. Meyers, R. Lindborg, and M. Gullström. 2017. Temperate fish community variation over seasons in relation to large-scale geographic seascape variables. Canadian Journal of Fisheries and Aquatic Sciences 75: 1723–1732.
R Core Team. 2021. R: A language and environment for statistical computing. Vienna: Austria.
Ramage, D.L., and D.R. Schiel. 1998. Reproduction in the seagrass Zostera novazelandica on intertidal platforms in southern New Zealand. Marine Biology 130: 479–489. https://doi.org/10.1007/s002270050268.
Ray, B.R., M.W. Johnson, K. Cammarata, and D.L. Smee. 2014. Changes in seagrass species composition in northwestern Gulf of Mexico estuaries: Effects on associated seagrass fauna. PLoS ONE 9 (9): e107751.
Rees, M.J., N.A. Knott, and A.R. Davis. 2018. Habitat and seascape patterns drive spatial variability in temperate fish assemblages: Implications for marine protected areas. Marine Ecology Progress Series 607: 171–186.
Ribeiro, J., L. Bentes, R. Coelho, J. Gonçalves, P. Lino, P. Monteiro, and K. Erzini. 2006. Seasonal, tidal and diurnal changes in fish assemblages in the Ria Formosa lagoon (Portugal). Estuarine, Coastal and Shelf Science 67: 461–474. https://doi.org/10.1016/j.ecss.2005.11.036.
Ripley, B., and W. Venables. 2021. Feed-forward neural networks and multinomial log-linear models. R (version 7.3–16).
Robertson, D.A. 1973. Planktonic eggs and larvae of some New Zealand marine teleosts. PhD Thesis, University of Otago.
Ruiz-Frau, A., S. Gelcich, I.E. Hendriks, C.M. Duarte, and N. Marbà. 2017. Current state of seagrass ecosystem services: Research and policy integration. Ocean & Coastal Management 149: 107–115. https://doi.org/10.1016/j.ocecoaman.2017.10.004.
Scapin, L., F. Cavraro, S. Malavasi, F. Riccato, M. Zucchetta, and P. Franzoi. 2018. Linking pipefishes and seahorses to seagrass meadows in the Venice lagoon: Implications for conservation. Aquatic Conservation: Marine and Freshwater Ecosystems 28: 282–295. https://doi.org/10.1002/aqc.2860.
Schaffmeister, B.E., J.G. Hidding, and W.J. Wolff. 2006. Habitat use of shrimps in the intertidal and shallow subtidal seagrass beds of the tropical Banc d’Arguin, Mauritania. Journal of Sea Research 55: 230–243.
Schwarz, A.-M., M. Morrison, I. Hawes, and J. Halliday. 2006. Physical and biological characteristics of a rare marine habitat: sub-tidal seagrass beds of offshore islands. Science for Conservation, Department of Conservation 269: 30.
Sharma, S., J. Goff, R.M. Moody, D. Byron, K.L. Heck, S.P. Powers, C. Ferraro, and J. Cebrian. 2016. Do restored oyster reefs benefit seagrasses? An experimental study in the Northern Gulf of Mexico: Oyster reef restoration and seagrasses. Restoration Ecology 24: 306–313. https://doi.org/10.1111/rec.12329.
Shibuno, T., Y. Nakamura, M. Horinouchi, and M. Sano. 2008. Habitat use patterns of fishes across the mangrove-seagrass-coral reef seascape at Ishigaki Island, southern Japan. Ichthyological Research 55: 218–237. https://doi.org/10.1007/s10228-007-0022-1.
Shokri, M.R., W. Gladstone, and J. Jelbart. 2009. The effectiveness of seahorses and pipefish (Pisces: Syngnathidae) as a flagship group to evaluate the conservation value of estuarine seagrass beds. Aquatic Conservation: Marine and Freshwater Ecosystems 19: 588–595. https://doi.org/10.1002/aqc.1009.
Short, F., T. Carruthers, W. Dennison, and M. Waycott. 2007. Global seagrass distribution and diversity: A bioregional model. Journal of Experimental Marine Biology and Ecology 350: 3–20. https://doi.org/10.1016/j.jembe.2007.06.012.
Smale, D.A., T. Wernberg, E.C.J. Oliver, M. Thomsen, B.P. Harvey, S.C. Straub, M.T. Burrows, et al. 2019. Marine heatwaves threaten global biodiversity and the provision of ecosystem services. Nature Climate Change 9: 306–312. https://doi.org/10.1038/s41558-019-0412-1.
Smith, T.M., J.S. Hindell, G.P. Jenkins, R.M. Connolly, and M.J. Keough. 2011. Fine-scale spatial and temporal variations in diets of the pipefish Stigmatopora nigra within seagrass patches. Journal of Fish Biology 78: 1824–1832. https://doi.org/10.1111/j.1095-8649.2011.02977.x.
Spalding, M., M. Taylor, C. Ravilious, F. Short, and E. Green. 2003. The distribution and status of seagrasses. In World Atlas of Seagrasses 5–26.
Tan, Y.M., O. Dalby, G.A. Kendrick, J. Statton, E.A. Sinclair, M.W. Fraser, P.I. Macreadie, et al. 2020. Seagrass restoration is possible: Insights and lessons from Australia and New Zealand. Frontiers in Marine Science 7: 617. https://doi.org/10.3389/fmars.2020.00617.
Tarimo, B., M. Winder, M.S.P. Mtolera, C.A. Muhando, and M. Gullström. 2022. Seasonal distribution of fish larvae in mangrove-seagrass seascapes of Zanzibar (Tanzania). Scientific Reports 12: 4196.
Taylor, M.D., B. Fry, A. Becker, and N. Moltschaniwskyj. 2017. Recruitment and connectivity influence the role of seagrass as a penaeid nursery habitat in a wave dominated estuary. Science of the Total Environment 584–585: 622–630.
Thomsen, M.S., A. Moser, M. Pullen, D. Gerber, and S.P. Flanagan. 2020. Seagrass beds provide habitat for crabs, shrimps and fish in two estuaries on the South Island of New Zealand. ECan Report.
Thomsen, M.S., and T. Wernberg. 2015. The devil in the detail: Harmful seaweeds are not harmful to everyone. Global Change Biology 21: 1381–1382. https://doi.org/10.1111/gcb.12772.
Thomsen, M.S., T. Wernberg, A.H. Engelen, F. Tuya, M.A. Vanderklift, M. Holmer, K.J. McGlathery, F. Arenas, J. Kotta, and B.R. Silliman. 2012. A meta-analysis of seaweed impacts on seagrasses: generalities and knowledge gaps. PLOS ONE 7. Public Library of Science: e28595. https://doi.org/10.1371/journal.pone.0028595.
Turner, S., and A.-M. Schwarz. 2006. Management and conservation of seagrass in New Zealand: an introduction. 264. Science for Conservation. New Zealand Department of Conservation.
Uhrin, A.V., and J.G. Holmquist. 2003. Effects of propeller scarring on macrofaunal use of the seagrass Thalassia testudinum. Marine Ecology Progress Series 250: 61–70.
van Houte-Howes, K.S.S., S.J. Turner, and C.A. Pilditch. 2004. Spatial differences in macroinvertebrate communities in intertidal seagrass habitats and unvegetated sediment in three New Zealand estuaries. Estuaries 27: 945–957. https://doi.org/10.1007/BF02803421.
Watson, R.A., R.G. Coles, and W.J. Lee Long. 1993. Simulation estimates of annual yield and landed value for commercial penaeid prawns from a tropical seagrass habitat, Northern Queensland, Australia. Australian Journal of Marine and Freshwater Research 44: 211–219.
Waycott, M., C.M. Duarte, T.J.B. Carruthers, R.J. Orth, W.C. Dennison, S. Olyarnik, A. Calladine, et al. 2009. Accelerating loss of seagrasses across the globe threatens coastal ecosystems. Proceedings of the National Academy of Sciences 106: 12377–12381. https://doi.org/10.1073/pnas.0905620106.
Webb, B.F. 1972. Fish populations of the Avon‐Heathcote estuary. New Zealand Journal of Marine and Freshwater Research 6. Taylor & Francis: 570–601. https://doi.org/10.1080/00288330.1972.9515447.
Webb, B.F. 1973. Fish populations of the Avon-Heathcote Estuary: 2. Breeding and Gonad Maturity. New Zealand Journal of Marine and Freshwater Research 7: 45–66. https://doi.org/10.1080/00288330.1973.9515455.
Włodarska-Kowalczuk, M., E. Jankowska, L. Kotwicki, and P. Balazy. 2014. Evidence of season-dependency in vegetation effects on macrofauna in temperate seagrass meadows (Baltic Sea). PLOS ONE 9. Public Library of Science: e100788. https://doi.org/10.1371/journal.pone.0100788.
Zeileis, A., C. Kleiber, and S. Jackman. 2008. Regression models for count data in R. Journal of Statistical Software 27.
Acknowledgements
This research was conducted in the Akaroa Taiāpure, within the rohe of Ōnuku Rūnanga, which represents Ngāi Tārewa and Ngāti Īrakehu, whom we thank for their support of this research. We acknowledge Ngāi Tūāhuriri upon whose lands the analyses and writing was conducted. This research was primarily funded by the Brian Mason Trust (grant number 201824) with supplemental funds provided by the University of Canterbury. Thanks to Averill Moser-Rust for her assistance with fieldwork and to Environment Canterbury for funding her summer scholarship. Additional field assistance was provided by Renny Bishop and Kyle Andales. All research was conducted under the MPI Special Permit numbers 590 (amendment 2) and 728, and we thank Dr. John Pirker for managing and supporting those permit applications. Sincere thanks to Dr. Zach Marion for his lively discussions about the statistical analyses conducted, for sharing his code, and for feedback on early drafts of the manuscript. We also extend our thanks to Dr. Warwick Allen, Dr. Will Godsoe, and Professor James Fordyce for feedback on early drafts of this work, and two anonymous reviewers and an editor for helping improve previous versions of the manuscript.
Funding
Open Access funding enabled and organized by CAUL and its Member Institutions.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by Ronald Baker
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Pullen, M., Gerber, D., Thomsen, M.S. et al. Seasonal Dynamics of Faunal Diversity and Population Ecology in an Estuarine Seagrass Bed. Estuaries and Coasts 45, 2578–2591 (2022). https://doi.org/10.1007/s12237-022-01103-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12237-022-01103-8