Abstract
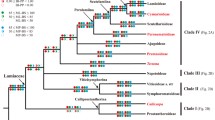
Chloranthaceae were one of the first common lines during the early radiation of angiosperms, possibly reflecting adaptation to more open habitats. Phylogenetic analyses clarify the position of Cretaceous mesofossils in molecular trees of Recent taxa. Plants that produced Asteropollis pollen, with tepals adnate to a single carpel, are nested in crown group Chloranthaceae with Hedyosmum; Canrightiopsis, with three stamens and no perianth, is sister to Sarcandra and Chloranthus; and Canrightia is a stem relative that illustrates a still bisexual stage in floral reduction. Plants that produced Pennipollis pollen are related to Chloranthaceae and/or Ceratophyllum rather than monocots. Appomattoxia, which produced Tucanopollis pollen, has equivocal affinities, but Pseudoasterophyllites, with similar pollen and stems with reduced leaves, may be a link between Chloranthaceae and Ceratophyllum. These results imply that flowers became unisexual before losing the perianth, while bisexual flowers in Canrightiopsis, Sarcandra, and Chloranthus are secondarily derived from unisexual flowers.














Similar content being viewed by others
References
Antonelli, A. & I. Sanmartín. 2011. Mass extinction, gradual cooling, or rapid radiation? Reconstructing the spatiotemporal evolution of the ancient angiosperm genus Hedyosmum (Chloranthaceae) using empirical and simulated approaches. Systematic Biology 60: 596–615.
Antonov A. S., A. V. Troitsky, T. K. Samigullin, V. K. Bobrova, K. M. Valiejo-Roman & W. Martin. 2000. Early events in the evolution of angiosperms deduced from cp rDNA ITS 2–4 sequence comparisons. Pp. 210–214. In: Y.-H. Liu, H.-M. Fan, Z.-Y. Chen, Q.-G. Wu & Q.-W. Zeng (eds.), Proceedings of the International Symposium on the Family Magnoliaceae. Science Press, Beijing.
Batten, D. J. & A. M. Zavattieri. 1995. Occurrence of dispersed seed cuticles and similar microfossils in mainly Cretaceous successions of the Northern Hemisphere. Cretaceous Research 16: 73-94.
Blanc, P. 1986. Edification d'arbres par croissance d'établissement de type monocotylédonien: l'exemple de Chloranthaceae. Pp. 101-123. In: Colloque international sur l'Arbre 1986. Naturalia Monspeliensia, numéro hors série.
Brenner, G. J. 1963. The spores and pollen of the Potomac Group of Maryland. Maryland Department of Geology, Mines and Water Resources Bulletin 27: 1-215.
--- 1976. Middle Cretaceous floral provinces and early migrations of angiosperms. Pp. 23-47. In: C. B. Beck (ed.), Origin and early evolution of angiosperms. Columbia University Press, New York.
---. 1996. Evidence for the earliest stage of angiosperm pollen evolution: a paleoequatorial section from Israel. Pp. 91-115. In: D. W. Taylor & L. J. Hickey (eds.), Flowering plant origin, evolution & phylogeny. Chapman & Hall, New York.
Burger, W. C. 1977. The Piperales and the monocots. Alternate hypotheses for the origin of monocotyledonous flowers. Botanical Review 43: 345-393.
Burrows, C. J. 1996. Germination behaviour of seeds of the New Zealand woody species Ascarina lucida, Coprosma grandifolia, Melicytus lanceolatus, and Solanum laciniatum. New Zealand Journal of Botany 34: 509-515.
Cantino, P. D., J. A. Doyle, S. W. Graham, W. S. Judd, R. G. Olmstead, D. E. Soltis, P. S. Soltis & M. J. Donoghue. 2007. Towards a phylogenetic nomenclature of Tracheophyta. Taxon 56: 822-846.
Clarke, J. T., R. C. M. Warnock & P. C. J. Donoghue. 2011. Establishing a time-scale for plant evolution. New Phytologist 192: 266-301.
Cordemoy, C. J. de . 1863. Monographie du groupe des Chloranthacées. Adansonia 3: 280–310.
Couper, R. A. 1958. British Mesozoic microspores and pollen grains. Palaeontographica Abteilung B 103: 75-179.
Crane, P. R., E. M. Friis & K. R. Pedersen. 1989. Reproductive structure and function in Cretaceous Chloranthaceae. Plant Systematics and Evolution 165: 211-226.
Crane, P. R., K. R. Pedersen, E. M. Friis & A. N. Drinnan. 1993. Early Cretaceous (early to middle Albian) platanoid inflorescences associated with Sapindopsis leaves from the Potomac Group of eastern North America. Systematic Botany 18: 328-344.
D’Arcy, W. G. & R. L. Liesner. 1981. Hedyosmum (Chloranthaceae) in Panama. Systematic Botany 6: 74-86.
Dickison, W. C. 1992. Morphology and anatomy of the flower and pollen of Saruma henryi Oliv., a phylogenetic relict of the Aristolochiaceae. Bulletin of the Torrey Botanical Club 119: 392-400.
Doria, M. G., N. Pabón-Mora & F. González. 2012. Reassessing inflorescence and floral morphology and development in Hedyosmum (Chloranthaceae). International Journal of Plant Sciences 173: 735-750.
Doyle, J. A. 1969. Cretaceous angiosperm pollen of the Atlantic Coastal Plain and its evolutionary significance. Journal of the Arnold Arboretum 50: 1-35.
---. 1992. Revised palynological correlations of the lower Potomac Group (USA) and the Cocobeach sequence of Gabon (Barremian-Aptian). Cretaceous Research 13: 337-349.
---. 2001. Significance of molecular phylogenetic analyses for paleobotanical investigations on the origin of angiosperms. The Palaeobotanist 50: 167-188.
---. 2005. Early evolution of angiosperm pollen as inferred from molecular and morphological phylogenetic analyses. Grana 44: 227-251.
--- & P. K. Endress. 2000. Morphological phylogenetic analysis of basal angiosperms: comparison and combination with molecular data. International Journal of Plant Sciences 161(Supplement): S121-S153.
--- & ---. 2010. Integrating Early Cretaceous fossils into the phylogeny of living angiosperms: Magnoliidae and eudicots. Journal of Systematics and Evolution 48: 1-35.
--- & ---. 2011. Tracing the early evolutionary diversification of the angiosperm flower. Pp. 88-119. In: L. Wanntorp & L. P. Ronse De Craene (eds.), Flowers on the tree of life. Cambridge University Press, Cambridge, UK.
--- & ---. 2014. Integrating Early Cretaceous fossils into the phylogeny of living angiosperms: ANITA lines and relatives of Chloranthaceae. International Journal of Plant Sciences 175: 555-600.
--- & L. J. Hickey. 1976. Pollen and leaves from the mid-Cretaceous Potomac Group and their bearing on early angiosperm evolution. Pp. 139-206. In: C. B. Beck (ed.), Origin and early evolution of angiosperms. Columbia University Press, New York.
--- & C. L. Hotton. 1991. Diversification of early angiosperm pollen in a cladistic context. Pp. 169-195. In: S. Blackmore & S. H. Barnes (eds.), Pollen and spores: patterns of diversification. Clarendon Press, Oxford.
--- & G. R. Upchurch, Jr. 2014. Angiosperm clades in the Potomac Group: what have we learned since 1977? Bulletin of the Peabody Museum of Natural History 55: 111-134.
---, P. Biens, A. Doerenkamp & S. Jardiné. 1977. Angiosperm pollen from the pre-Albian Cretaceous of Equatorial Africa. Bulletin des Centres de Recherches Exploration-Production Elf-Aquitaine 1: 451-473.
---, H. Eklund & P. S. Herendeen. 2003. Floral evolution in Chloranthaceae: implications of a morphological phylogenetic analysis. International Journal of Plant Sciences 164(5 Supplement): S365-S382.
---, P. K. Endress & G. R. Upchurch, Jr. 2008. Early Cretaceous monocots: a phylogenetic evaluation. Acta Musei Nationalis Pragae, Series B, Historia Naturalis, 64(2-4): 59-87.
---, S. Jardiné & A. Doerenkamp. 1982. Afropollis, a new genus of early angiosperm pollen, with notes on the Cretaceous palynostratigraphy and paleoenvironments of Northern Gondwana. Bulletin des Centres de Recherches Exploration-Production Elf-Aquitaine 6: 39-117.
---. M. Van Campo & B. Lugardon. 1975. Observations on exine structure of Eucommiidites and Lower Cretaceous angiosperm pollen. Pollen et Spores 17: 429-486.
Drinnan, A. N., P. R. Crane, E. M. Friis & K. R. Pedersen. 1990. Lauraceous flowers from the Potomac Group (mid-Cretaceous) of eastern North America. Botanical Gazette 151: 370-384.
---, ---, --- & ---. 1991. Angiosperm flowers and tricolpate pollen of buxaceous affinity from the Potomac Group (mid-Cretaceous) of eastern North America. American Journal of Botany 78: 153-176.
Duvall, M. R., S. Mathews, N. Mohammad & T. Russell. 2006. Placing the monocots: conflicting signal from trigenomic analyses. Aliso 22: 79-90.
---, J. W. Robinson, J. G. Mattson & A. Moore. 2008. Phylogenetic analyses of two mitochondrial metabolic genes sampled in parallel from angiosperms find fundamental interlocus incongruence. American Journal of Botany 95: 871-884.
Eklund, H. 1999. Phylogeny of living and fossil Chloranthaceae. Big survivors with small flowers: fossil history and evolution of Laurales and Chloranthaceae. PhD Thesis, Uppsala University, Sweden
---, J. A. Doyle & P. S. Herendeen. 2004. Morphological phylogenetic analysis of living and fossil Chloranthaceae. International Journal of Plant Sciences 165: 107-151.
---, E. M. Friis & K. R. Pedersen. 1997. Chloranthaceous floral structures from the Late Cretaceous of Sweden. Plant Systematics and Evolution 207: 13-42.
Endress, P. K. 1986. Reproductive structures and phylogenetic significance of extant primitive angiosperms. Plant Systematics and Evolution 152: 1-28.
--- 1987. The Chloranthaceae: reproductive structures and phylogenetic position. Botanische Jahrbücher für Systematik 109: 153-226.
--- 1994. Evolutionary aspects of the floral structure in Ceratophyllum. Plant Systematics and Evolution Supplement 8: 175-183.
--- 2004. Structure and relationships of basal relictual angiosperms. Australian Systematic Botany 17: 343-366.
--- & F. B. Sampson. 1983. Floral structure and relationships of the Trimeniaceae (Laurales). Journal of the Arnold Arboretum 64: 447-473.
--- & A. Igersheim. 2000. The reproductive structures of the basal angiosperm Amborella trichopoda (Amborellaceae). International Journal of Plant Sciences 161 (Supplement): S237-S248.
--- & J. A. Doyle. 2009. Reconstructing the ancestral angiosperm flower and its initial specializations. American Journal of Botany 96: 22-66.
Feild, T. S., N. C. Arens, J. A. Doyle, T. E. Dawson & M. J. Donoghue. 2004. Dark and disturbed: a new image of early angiosperm ecology. Paleobiology 30: 82-107.
---, D. S. Chatelet & T. J. Brodribb. 2009. Ancestral xerophobia: a hypothesis on the whole plant ecophysiology of early angiosperms. Geobiology 7: 237-264.
Friedman, J. & S. C. H. Barrett. 2008. A phylogenetic analysis of the evolution of wind pollination in the angiosperms. International Journal of Plant Sciences 169: 49-58.
Friis, E. M. & K. R. Pedersen. 2011. Canrightia resinifera gen. et sp. nov., a new extinct angiosperm with Retimonocolpites-type pollen from the Early Cretaceous of Portugal: missing link in the eumagnoliid tree? Grana 50: 3-29.
---, P. R. Crane & K. R. Pedersen. 1986. Floral evidence for Cretaceous chloranthoid angiosperms. Nature 320: 163-164.
---, --- & ---. 1988. Reproductive structures of Cretaceous Platanaceae. Biologiske Skrifter Danske Videnskabernes Selskab 31: 1-55.
---, --- & ---. 2011. Early flowers and angiosperm evolution. Cambridge University Press, Cambridge.
---, H. Eklund, K. R. Pedersen & P. R. Crane. 1994a. Virginianthus calycanthoides gen. et sp. nov. – a calycanthaceous flower from the Potomac Group (Early Cretaceous) of eastern North America. International Journal of Plant Sciences 155: 772-785.
---, K. R. Pedersen & P. R. Crane. 1994b. Angiosperm floral structures from the Early Cretaceous of Portugal. Plant Systematics and Evolution Supplement 8: 31-49.
---, --- & ---. 1995. Appomattoxia ancistrophora gen. et sp. nov., a new Early Cretaceous plant with similarities to Circaeaster and extant Magnoliidae. American Journal of Botany 82: 933-943.
---, --- & ---. 1999. Early angiosperm diversification: the diversity of pollen associated with angiosperm reproductive structures in Early Cretaceous floras from Portugal. Annals of the Missouri Botanical Garden 86: 259-296.
---, --- & ---. 2000a. Fossil floral structures of a basal angiosperm with monocolpate, reticulate-acolumellate pollen from the Early Cretaceous of Portugal. Grana 39: 226-239.
---, --- & ---. 2000b. Reproductive structure and organization of basal angiosperms from the Early Cretaceous (Barremian or Aptian) of western Portugal. International Journal of Plant Sciences 161 (Supplement): S169-S182.
---, --- & ---. 2001. Fossil evidence of water lilies (Nymphaeales) in the Early Cretaceous. Nature 410: 357-360.
---, --- & ---. 2006. Cretaceous angiosperm flowers: innovation and evolution in plant reproduction. Palaeogeography Palaeoclimatology Palaeoecology 232: 251-293.
---, --- & ---. 2010. Cretaceous diversification of angiosperms in the western part of the Iberian Peninsula. Review of Palaeobotany and Palynology 162: 341-361.
---, ---, M. von Balthazar, G. W. Grimm & P. R. Crane. 2009. Monetianthus mirus gen. et sp. nov., a nymphaealean flower from the Early Cretaceous of Portugal. International Journal of Plant Sciences 170: 1086–1101.
---, G. W. Grimm, M. M. Mendes & K. R. Pedersen. 2015. Canrightiopsis, a new Early Cretaceous fossil with Clavatipollenites-type pollen bridge the gap between extinct Canrightia and extant Chloranthaceae. Grana 54: 184-212.
Goldberg, E. E., S. P. Otto, J. C. Vamosi, I. Mayrose, N. Sabath, R. Ming & T.-L. Ashman. 2017. Macroevolutionary synthesis of flowering plant sexual systems. Evolution 71: 898-912.
Gomez, B., V. Daviero-Gomez, C. Coiffard, C. Martín-Closas & D. L. Dilcher. 2015. Montsechia, an ancient aquatic angiosperm. Proceedings of the National Academy of Sciences of the USA 112: 10985-10988.
Góczán, F. & M. Juhász. 1984. Monosulcate pollen grains of angiosperms from Hungarian Albian sediments I. Acta Botanica Hungarica 30: 289-319.
Hartkopf-Fröder, C., J. Rust, T. Wappler, E. M. Friis & A. Viehofen. 2012. Mid-Cretaceous charred fossil flowers reveal direct observation of arthropod feeding strategies. Biology Letters 8: 295-298.
Hedlund, R. W. & G. Norris. 1968. Spores and pollen grains from Fredericksburgian (Albian) strata, Marshall County, Oklahoma. Pollen et Spores 10: 129-159.
Heimhofer, U. & P. A. Hochuli. 2010. Early Cretaceous angiosperm pollen from a low-latitude succession (Araripe Basin, NE Brazil). Review of Palaeobotany and Palynology 161: 105-126.
---, ---, S. Burla & H. Weissert. 2007. New records of Early Cretaceous angiosperm pollen from Portuguese coastal deposits: implications for the timing of the early angiosperm radiation. Review of Palaeobotany and Palynology 144: 39-76.
Herendeen, P. S., W. L. Crepet & K. C. Nixon. 1993. Chloranthus-like stamens from the Upper Cretaceous of New Jersey. American Journal of Botany 80: 865-871.
Hermsen, E. J. & J. R. Hendricks. 2008. W(h)ither fossils? Studying morphological character evolution in the age of molecular sequences. Annals of the Missouri Botanical Garden 95: 72-100.
Hesse, M. 2001. Pollen characters of Amborella trichopoda (Amborellaceae): a reinvestigation. International Journal of Plant Sciences 162: 201-208.
--- & R. Zetter. 2007. The fossil pollen record of Araceae. Plant Systematics and Evolution 263: 93-115.
Hickey, L. J. & J. A. Doyle. 1977. Early Cretaceous fossil evidence for angiosperm evolution. Botanical Review 43: 1-104.
--- & J. A. Wolfe. 1975. The bases of angiosperm phylogeny: vegetative morphology. Annals of the Missouri Botanical Garden 62: 538-589.
Hughes, N. F. 1994. The enigma of angiosperm origins. Cambridge University Press, Cambridge, UK.
--- & A. B. McDougall. 1987. Records of angiospermid pollen entry into the English Early Cretaceous succession. Review of Palaeobotany and Palynology 50: 255-272.
---, G. E. Drewry & J. F. Laing. 1979. Barremian earliest angiosperm pollen. Palaeontology 22: 513-535.
Iwamoto, A., A. Shimizu & H. Ohba. 2003. Floral development and phyllotactic variation in Ceratophyllum demersum (Ceratophyllaceae). American Journal of Botany 90: 1124-1130.
Jansen, R. K., Z. Cai, L. A. Raubeson, H. Daniell, C. W. de Pamphilis, J. Leebens-Mack, K. F. Müller, M. Guisinger-Bellian, R. C. Haberle, A. K. Hansen, T. W. Chumley, S.-B. Lee, R. Peery, J. R. McNeal, J. V. Kuehl & J. L. Boore. 2007. Analysis of 81 genes from 64 plastid genomes resolves relationships in angiosperms and identifies genome-scale evolutionary patterns. Proceedings of the National Academy of Sciences of the USA 104: 19369-19374.
Jérémie, J. 1980. Notes sur le genre Ascarina (Chloranthaceae) en Nouvelle-Calédonie et à Madagascar. Adansonia, Sér. 2 20: 273-285.
Kemp, E. M. 1968. Probable angiosperm pollen from British Barremian to Albian strata. Palaeontology 11: 421-434.
Krassilov, V. 2011. On Montsechia, an angiospermoid plant from the Lower Cretaceous of Las Hoyas, Spain: new data and interpretations. Acta Palaeobotanica 51: 181-205.
Krutzsch, W. 1989. Paleogeography and historical phytogeography (paleochorology) in the Neophyticum. Plant Systematics and Evolution 162: 5-61.
Kvaček, J. & H. Eklund. 2003. A report on newly recovered reproductive structures from the Cenomanian of Bohemia (Central Europe). International Journal of Plant Sciences 164: 1021-1039.
--- & E. M. Friis. 2010. Zlatkocarpus gen. nov., a new angiosperm reproductive structure with monocolpate-reticulate pollen from the Late Cretaceous (Cenomanian) of the Czech Republic. Grana 49: 115-127.
---, B. Gomez & R. Zetter. 2012. The early angiosperm Pseudoasterophyllites cretaceus from Albian-Cenomanian of Czech Republic and France revisited. Acta Palaeontologica Polonica 57: 437-443.
---, J. A. Doyle, P. K. Endress, V. Daviero-Gomez, B. Gomez & M. Tekleva. 2016. Pseudoasterophyllites cretaceus from the Cenomanian (Cretaceous) of the Czech Republic: A possible link between Chloranthaceae and Ceratophyllum. Taxon 65: 1345–1373.
Leroy, J.-F. 1983. The origin of angiosperms: an unrecognized ancestral dicotyledon, Hedyosmum (Chloranthales), with a strobiloid flower is living today. Taxon 32: 169-175.
Loconte, H. & D. W. Stevenson. 1991. Cladistics of the Magnoliidae. Cladistics 7: 267-296.
Luo, Y.-B. & Z.-Y. Li. 1999. Pollination ecology of Chloranthus serratus (Thunb.) Roem. et Schult. and Ch. fortunei (A. Gray) Solms-Laub. (Chloranthaceae). Annals of Botany 83: 489-499.
Ma, S.-B., Y.-H. Wang & M.-K. Cui. 1997. A contribution to the reproductive biology of Chloranthus holostagius (Chloranthaceae) in Mile population. Acta Botanica Yunnanica 19: 415-422.
Maddison, D. R. & W. P. Maddison. 2003. MacClade 4: analysis of phylogeny and character evolution, version 4.06. Sinauer Associates, Sunderland, Mass.
Martin, T. J. & J. Ogden. 2002. The seed ecology of Ascarina lucida: a rare New Zealand tree adapted to disturbance. New Zealand Journal of Botany 40: 397-404.
Martín-Closas, C. 2003. The fossil record and evolution of freshwater plants: a review. Geologica Acta 1: 315-338.
Martínez, C., S. Madriñán, M. Zavada & C. A. Jaramillo. 2013. Tracing the fossil pollen record of Hedyosmum (Chloranthaceae), an old lineage with recent Neotropical diversification. Grana 52: 161-180.
Massoni, J., J. Doyle & H. Sauquet. 2015. Fossil calibration of Magnoliidae, an ancient lineage of angiosperms. Palaeontologia Electronica 17(3), 2FC, 25 pp.
Mathews, S. & M. J. Donoghue. 1999. The root of angiosperm phylogeny inferred from duplicate phytochrome genes. Science 286: 947-950.
McGlone, M. S. & N. T. Moar. 1977. The Ascarina decline and post-glacial climatic change in New Zealand. New Zealand Journal of Botany 15: 485-489.
Meeuse, A. D. J. 1972. Facts and fiction in floral morphology with special reference to the Polycarpicae. Acta Botanica Neerlandica 21: 113-127, 235-252, 351-365.
Miner, E. L. 1935. Paleobotanical examinations of Cretaceous and Tertiary coals. American Midland Naturalist 16: 585-625.
Moore, L. B. 1977. The flowers of Ascarina lucida Hook. f. (Chloranthaceae). New Zealand Journal of Botany 15: 491-494.
Moore, M. J., C. D. Bell, P. S. Soltis & D. E. Soltis. 2007. Using plastid genome-scale data to resolve enigmatic relationships among basal angiosperms. Proceedings of the National Academy of Sciences of the USA 104: 19363-19368.
---, N. Hassan, M. A. Gitzendanner, R. A. Bruenn, M. Croley, A. Vandeventer, J. W. Horn, A. Dhingra, S. F. Brockington, M. Latvis, J. Ramdial, R. Alexandre, A. Piedrahita, Z. Xi, C. C. Davis, P. S. Soltis & D. E. Soltis. 2011. Phylogenetic analysis of the plastid inverted repeat for 244 species: insights into deeper-level angiosperm relationships from a long, slowly evolving sequence region. International Journal of Plant Sciences 172: 541–558.
Muller, J. 1981. Fossil pollen records of extant angiosperms. Botanical Review 47: 1-142.
Nixon, K. C. 2008. Paleobotany, evidence, and molecular dating: an example from the Nymphaeales. Annals of the Missouri Botanical Garden 95: 43-50.
---, W. L. Crepet, D. Stevenson & E. M. Friis. 1994. A reevaluation of seed plant phylogeny. Annals of the Missouri Botanical Garden 81: 484-533.
Norris, G. 1967. Spores and pollen from the Lower Colorado Group (Albian-?Cenomanian) of central Alberta. Palaeontographica Abteilung B 120: 72-115.
Ogg, J. G. & L. A. Hinnov. 2013. Cretaceous. Pp. 793-853. In: F. M. Gradstein, J. G. Ogg, M. D. Schmitz & G. M. Ogg (eds.), The geologic time scale 2012. Elsevier, Amsterdam.
Parkinson, C. L., K. L. Adams & J. D. Palmer. 1999. Multigene analyses identify the three earliest lineages of extant flowering plants. Current Biology 9: 1485-1488.
Pedersen, K. R., P. R. Crane, A. N. Drinnan & E. M. Friis. 1991. Fruits from the mid-Cretaceous of North America with pollen grains of the Clavatipollenites type. Grana 30: 577-590.
Penny, J. H. J. 1988. Early Cretaceous acolumellate semitectate pollen from Egypt. Palaeontology 31: 373-418.
Pierce, R. L. 1961. Lower Upper Cretaceous plant microfossils from Minnesota. Minnesota Geological Survey Bulletin 42: 1-86.
Qiu, Y.-L., J. Lee, F. Bernasconi-Quadroni, D. E. Soltis, P. S. Soltis, M. Zanis, E. A. Zimmer, Z. Chen, V. Savolainen & M. W. Chase. 1999. The earliest angiosperms: evidence from mitochondrial, plastid and nuclear genomes. Nature 402: 404-407.
---, L Li, B. Wang, J.-Y. Xue, T. A. Hendry, R.-Q. Li, J. W. Brown, Y. Liu, G. T. Hudson & Z.-D. Chen. 2010. Angiosperm phylogeny inferred from sequences of four mitochondrial genes. Journal of Systematics and Evolution 48: 391-425.
Rawlings, G. B. 1974. Northland notes 3. New Zealand Journal of Botany 12: 563-565.
Regal, P. J. 1982. Pollination by wind and animals: ecology of geographic patterns. Annual Review of Ecology and Systematics 13: 497-524.
Regali, M. S. P. 1989. Tucanopollis, um gênero novo das angiospermas primitivas. Boletim de Geociências da Petrobrás 3, 395-402.
---, N. Uesugui & A. S. Santos. 1974. Palinologia dos sedimentos meso-cenozóicos do Brasil. Boletim Técnico da Petrobrás 17: 177-191, 263-301.
Saarela, J. M., H. S. Rai, J. A. Doyle, P. K. Endress, S. Mathews, A. D. Marchant, B. G. Briggs & S. W. Graham, 2007. Hydatellaceae identified as a new branch near the base of the angiosperm phylogenetic tree. Nature 446: 312-315.
Sauquet, H., M. von Balthazar, S. Magallón, J. A. Doyle, P. K. Endress, E. J. Bailes, E. Barroso de Morais, K. Bull-Hereñu, L. Carrive, M. Chartier, G. Chomicki, M. Coiro, R. Cornette, J. H. L. El Ottra, C. Epicoco, C. S. P. Foster, F. Jabbour, A. Haevermans, T. Haevermans, R. Hernández, S. A. Little, S. Löfstrand, J. A. Luna, J. Massoni, S. Nadot, S. Pamperl, C. Prieu, E. Reyes, P. dos Santos, K. M. Schoonderwoerd, S. Sontag, A. Soulebeau, Y. Staedler, G. F. Tschan, A. W.-S. Leung & J. Schönenberger, 2017. The ancestral flower of angiosperms and its early diversification. Nature Communications 8:16047, 10 pp.
Sender, L. M., J. A. Doyle, U. Villanueva-Amadoz, D. Pons, J. B. Diez & J. Ferrer. 2016. First records of the angiosperm genus Sapindopsis Fontaine (Platanaceae) in western Eurasia from middle to latest Albian deposits of Spain. Review of Palaeobotany and Palynology 230: 10-21.
Singh, C. 1971. Lower Cretaceous microfloras of the Peace River area, northwestern Alberta. Research Council of Alberta Bulletin 28: 1-310.
Smith, A. C. 1976. Studies of Pacific Island plants. XXXIII. The genus Ascarina (Chloranthaceae) in the southern Pacific. Journal of the Arnold Arboretum 57: 405-425.
---. 1981. Flora Vitiensis nova. A new flora of Fiji (Spermatophytes only) vol. 2. Pacific Tropical Botanical Garden, Lawai, Hawaii.
Smith, S. Y. & R. A. Stockey. 2007. Pollen morphology and ultrastructure of Saururaceae. Grana 46: 250-267.
Soltis, D. E., P. S. Soltis, P. K. Endress & M. W. Chase. 2005. Phylogeny and evolution of angiosperms. Sinauer Associates, Sunderland, Mass.
Soltis P. S., D. E. Soltis & M. W. Chase. 1999. Angiosperm phylogeny inferred from multiple genes as a tool for comparative biology. Nature 402: 402-404.
Springer, M. S., E. C. Teeling, O. Madsen, M. J. Stanhope & W. W. de Jong. 2001. Integrated fossil and molecular data reconstruct bat echolocation. Proceedings of the National Academy of Sciences of the USA 98: 6241-6246.
Strasburger, E. 1902. Ein Beitrag zur Kenntniss von Ceratophyllum submersum und phylogenetische Erörterungen. Jahrbücher für Wissenschaftliche Botanik 37: 477-526.
Sun, M., D. E. Soltis, P. S. Soltis, X. Zhu, J. G. Burleigh & Z. Chen. 2015. Deep phylogenetic incongruence in the angiosperm clade Rosidae. Molecular Phylogenetics and Evolution 83: 156-166.
Swamy, B. G. L. 1953. The morphology and relationships of the Chloranthaceae. Journal of the Arnold Arboretum 34: 375-408.
Takahashi, M. 1995. Development of structure-less pollen wall in Ceratophyllum demersum L. (Ceratophyllaceae). Journal of Plant Research 108: 205-208.
Tanrikulu, S., J. A. Doyle & I. Delusina. 2017. Early Cretaceous (Albian) spores and pollen from the Glen Rose Formation of Texas and their significance for correlation of the Potomac Group. Palynology. DOI:https://doi.org/10.1080/01916122.2017.1374309.
Taylor, D. W. & L. J. Hickey. 1992. Phylogenetic evidence for the herbaceous origin of angiosperms. Plant Systematics and Evolution 180: 137-156.
Thanikaimoni, G. 1985. Palynology and phylogeny, pp. 11-14 in van Bruggen, H. W. E., Monograph of the genus Aponogeton (Aponogetonaceae), Bibliotheca Botanica 137: 1-76.
Todzia, C. A. 1988. Chloranthaceae: Hedyosmum. Flora Neotropica Monograph 48: 1-139.
---. 1993. Chloranthaceae. Pp. 281-287. In: K. Kubitzki, J. G. Rohwer & V. Bittrich (eds.), The families and genera of vascular plants, volume II. Flowering plants. Dicotyledons. Magnoliid, hamamelid and caryophyllid families. Springer-Verlag, Berlin.
Tosaki, Y., H. Takahashi & S. S. Renner. 2001. Pollination of Sarcandra glabra (Chloranthaceae) in natural populations in Japan. Journal of Plant Research 114: 423-427.
Upchurch, G. R., Jr. 1984. Cuticular anatomy of angiosperm leaves from the Lower Cretaceous Potomac Group. I. Zone I leaves. American Journal of Botany 71: 192-202.
--- & D. L. Dilcher. 1990. Cenomanian angiosperm leaf megafossils, Dakota Formation, Rose Creek locality, Jefferson County, southeastern Nebraska. U. S. Geological Survey Bulletin 1915: 1-55.
van der Hammen, T. & E. Gonzalez. 1960. Upper Pleistocene and Holocene climate and vegetation of the “Sabana de Bogotá” (Colombia, South America). Leidse Geologische Mededelingen 25: 261-315.
von Balthazar, M. & P. K. Endress. 1999. Floral bract function, flowering process and breeding systems of Sarcandra and Chloranthus (Chloranthaceae). Plant Systematics and Evolution 218: 161-178.
Walker, J. W. & A. G. Walker. 1984. Ultrastructure of Lower Cretaceous angiosperm pollen and the origin and early evolution of flowering plants. Annals of the Missouri Botanical Garden 71: 464-521.
Wang, Y.-H., S.-B. Ma & M.-K. Cui. 1998. The characteristics of reproductive biology of Chloranthus holostagius. Journal of Yunnan University (Natural Sciences) 1998: S4.
---, K. Yang & S.-B. Ma. 1999. Reproductive biology of Chloranthus henryi (Chloranthaceae) in northeastern Yunnan. Acta Botanica Yunnanica 21: 218-224.
Ward, J. V. 1986. Early Cretaceous angiosperm pollen from the Cheyenne and Kiowa formations (Albian) of Kansas, U.S.A. Palaeontographica Abteilung B 202: 1-81.
Whitehead, D. R. 1969. Wind pollination in the angiosperms: evolutionary and environmental considerations. Evolution 23: 28-35.
Wilde, V., Z. Kvaček & J. Bogner. 2005. Fossil leaves of the Araceae from the European Eocene and notes on other aroid fossils. International Journal of Plant Sciences 166: 157-183.
Yoo, M.-J., C. D. Bell, P. S. Soltis & D. E. Soltis. 2005. Divergence times and historical biogeography of Nymphaeales. Systematic Botany 30: 693-704.
Zeng, L., Q. Zhang, R. Sun, H. Kong, N. Zhang & H. Ma. 2014. Resolution of deep angiosperm phylogeny using conserved nuclear genes and estimates of early divergence times. Nature Communications 5:4956, 12 pp.
Zhang, L.-B. & S. Renner. 2003. The deepest splits in Chloranthaceae as resolved by chloroplast sequences. International Journal of Plant Sciences 164 (Supplement): S383-S392.
Zhang, N., L. Zeng, H. Shan & H. Ma. 2012. Highly conserved low-copy nuclear genes as effective markers for phylogenetic analyses in angiosperms. New Phytologist 195: 923–937.
Zhang, Q., A. Antonelli, T. S. Feild & H.-Z. Kong. 2011. Revisiting taxonomy, morphological evolution, and fossil calibration strategies in Chloranthaceae. Journal of Systematics and Evolution 49: 315-329.
---, T. S. Feild & A. Antonelli. 2015. Assessing the impact of phylogenetic incongruence on taxonomy, floral evolution, biogeographical history, and phylogenetic diversity. American Journal of Botany 102: 566-580.
Acknowledgements
We thank Alejandra Gandolfo and Elizabeth Hermsen for inviting us to present a talk at the International Palaeontology Congress in Mendoza, Argentina (2014), on which this paper is based; Cuong Nguyen for hosting JAD at Cuc Phuong National Park; Paulo Schwirkowski for providing a photograph of Hedyosmum brasiliense; and two anonymous reviewers for useful comments on the manuscript.
Author information
Authors and Affiliations
Corresponding author
Appendix: Phylogenetic analysis of Canrightiopsis
Appendix: Phylogenetic analysis of Canrightiopsis
The data set used to evaluate the position of Canrightiopsis is that of Kvaček et al. (2016), including all potential fossil relatives of Chloranthaceae (except Chloranthistemon) treated in this paper (Table 1). Sources of data on other living and fossil taxa and the rationale for character definitions and scoring of difficult cases are discussed in Doyle and Endress (2000, 2010, 2014), Doyle et al. (2008), Endress and Doyle (2009), and Kvaček et al. (2016). In the following character list we have noted changes made between Doyle and Endress (2014) and Kvaček et al. (2016) that are most likely to affect inferred relationships of Chloranthaceae and potential relatives. We score Canrightiopsis as a consensus of the three species C. intermedia, C. crassitesta, and C. dinisii of Friis et al. (2015), whose preserved features do not differ in any of the characters in our data set; the differences among them are largely quantitative. These species are represented by isolated fruits with stamen scars (but no stamens), enclosed seeds, and adhering pollen.
Characters
Character states scored for Canrightia are indicated in bold font. Uncertain scorings (e.g., 0/1) are shown by putting both states in bold font. When no state is in bold font, the character is scored as unknown (including inapplicable).
1–41 Vegetative characters: unknown; see Kvaček et al. (2016).
42 Inflorescence (0) solitary flower (or occasionally with 1–2 lateral flowers), (1) botryoid, panicle, or thyrsoid (monotelic), (2) raceme, spike, or thyrse (polytelic).
43 Inflorescence partial units (0) single flowers, (1) cymes.
44 Inflorescence (or partial inflorescence) (0) not modified, (1) modified into globular head.
45 Pedicel (0) present in some or all flowers, (1) absent or highly reduced (flower sessile or subsessile).
46 Floral subtending bracts (0) present, (1) present in female, absent in male flowers, (2) absent in all flowers.
47 Sex of flowers (0) bisexual, (1) unisexual. Although Kvaček and Friis (2010) assumed that Zlatkocarpus had unisexual flowers, and we scored it accordingly in Doyle and Endress (2014), Friis et al. (2015) scored Zlatkocarpus as unknown. However, we assume that if Zlatkocarpus was bisexual there would be visible remnants or scars of stamens below or on the fruits, as there are in Canrightia, Canrightiopsis, Sarcandra, and Chloranthus.
48. Inflorescences of unisexual flowers (0) both sexes with more than one flower, (1) male with more than one flower, female with one flower (uniflorous, solitary). Bisexual taxa scored as unknown.
49 Floral base (0) no hypanthium, superior ovary, (1) hypanthium, superior, (2) inferior ovary. See Kvaček et al. (2016) for rescoring of Sarcandra and Chloranthus as inferior. Friis et al. (2015) used a character that distinguished partly epigynous from epigynous, but this is not likely to be informative for placement of Canrightiopsis among Chloranthaceae and related fossils, since only Hedyosmum and the Asteropollis plant are fully epigynous.
50 Floral receptacle (female portion) (0) short, (1) elongate.
51 Pits in receptacle bearing individual carpels (0) absent, (1) present.
52 Cortical vascular system (0) absent or supplying perianth only, (1) supplying androecium, (2) supplying androecium plus gynoecium.
53 Floral apex (0) used up after production of carpels, (1) protruding in mature flower. Not applicable in taxa with one carpel.
54 Perianth (0) present, (1) absent.
55–61 Perianth organization, features of perianth parts: inapplicable; see Kvaček et al. (2016).
62 Calyptra derived from the last one or two bracteate organs below the flower (0) absent, (1) present.
63 Stamen number (0) more than one, (1) one.
64 Androecium phyllotaxis (0) spiral, (1) whorled. Because of the uneven, one-sided position of the three stamens, we consider characters 64 and 65 inapplicable in Canrightiopsis.
65 Androecium merism (0) trimerous, (1) dimerous, (2) polymerous.
66 Stamen whorls (series when phyllotaxis is spiral; includes inner staminodes) (0) one, (1) two, (2) more than two. See Kvaček et al. (2016) for rescoring of Ascarina as unknown for characters 66 and 67.
67 Stamen positions (0) single, (1) double (at least in outer whorl). Inapplicable with one whorl of stamens.
68 Stamen fusion (0) free, (1) connate.
69 Inner staminodes (0) absent, (1) present. Inapplicable with one whorl of stamens; see Kvaček et al. (2016) for rescoring of Canrightia as unknown.
70–81 Stamen morphology, pollen development: unknown; see Kvaček et al. (2016).
82 Pollen unit (0) monads, (1) tetrads.
83 Pollen size (average) (0) large (> 50 μm), (1) medium (20–50 μm), (2) small (< 20 μm).
84 Pollen shape (0) boat-shaped, (1) globose, (2) triangular, angulaperturate.
85 Aperture type (0) single (presumably polar, including monosulcate and monoporate) or disulcate (one furrow at each pole), (1) inaperturate, (2) sulculate, (3) (syn)tricolpate with colpi arranged according to Garside’s law (with or without alternating colpi), (4) tricolpate.
86 Single aperture shape (0) elongate, (1) round.
87 Single aperture branching (0) unbranched, (1) with several branches.
88 Infratectum (0) granular (including “atectate”), (1) intermediate, (2) columellar; ordered.
89 Tectum (0) continuous or microperforate, (1) perforate (foveolate) to semitectate (e.g., reticulate), (2) reduced (not distinguishable from underlying granules).
90 Grading of reticulum (0) uniform, (1) finer at ends of sulcus (liliaceous), (2) finer at poles (rouseoid). Applicable only in taxa with state 1 in character 89.
91 Striate muri (0) absent, (1) present.
92 Supratectal spinules (smaller than the width of tectal muri in perforate and semitectate taxa; includes rounded as well as pointed elements) (0) absent, (1) present.
93 Prominent spines (larger than spinules, easily visible with light microscopy) (0) absent, (1) present.
94 Aperture membrane (0) smooth, (1) sculptured.
95 Extra-apertural nexine (0) foot layer, not consistently foliated, distinctly staining endexine absent or only discontinous traces, (1) foot layer and distinctly staining, continous endexine, or endexine only, (2) all or in part foliated, not distinctly staining.
96 Nexine thickness (0) absent or discontinuous traces, (1) thin but continuous, (2) thick (1/3 or more of total exine); ordered.
97 Carpel number (0) one, (1) two–five in one whorl or series (when spiral), (2) more than five in one whorl or series, (3) more than one whorl or series.
98 Carpel form (0) ascidiate up to stigma, (1) intermediate (both plicate and ascidiate zones below the stigma) with ovule(s) in the ascidiate zone, (2) completely plicate, or intermediate with some or all ovule(s) in the plicate zone. Because developmental or anatomical evidence is often needed to distinguish these states, we have scored this character as unknown in fossils, except when they have a clear ventral slit.
99 Postgenital sealing of carpel (0) none, (1) partial, (2) complete.
100 Secretion in area of carpel sealing (0) present, (1) absent.
101 Pollen tube transmitting tissue (0) not prominently differentiated, (1) one cell layer prominently differentiated, (2) more than one cell layer prominently differentiated.
102 Style (0) absent (stigma sessile or capitate), (1) present (elongated, distinctly constricted apical portion of carpel).
103 Stigma (0) extended (half or more of style-stigma zone), (1) restricted (above slit or around its upper part). In contrast to many fossils, the stigmatic zone in Canrightiopsis is distinct and similar to that in Sarcandra and Chloranthus.
104 Multicellular stigmatic protuberances or undulations (0) absent, (1) present. Characters 104 and 105 are not visible from the surface because of the abundant stigmatic secretion.
105 Stigmatic papillae (most elaborate type) (0) absent, (1) unicellular or with single emergent cell and one or more small basal cells, (2) uniseriate pluricellular with emergent portion consisting of two or more cells.
106 Extragynoecial compitum (0) absent, (1) present. Characters 106 and 107 are not applicable in unicarpellate taxa.
107 Carpel fusion (0) apocarpous, (1) parasyncarpous, (2) eusyncarpous (at least basally).
108 Oil cells in carpels (0) absent or internal, (1) intrusive. Inapplicable in taxa with no oil cells anywhere in the plant. Compared by Friis et al. (2015) with the intrusive oil cells of Chloranthus spicatus.
109 Long unicellular hairs on and/or between carpels (0) absent, (1) present. Characters 109–112 are usually not scored in fossils.
110 Short curved appressed unlignified hairs with up to two short basal cells and one long apical cell on carpels (0) absent, (1) present.
111 Nectary on dorsal or lateral sides of carpel or pistillode (0) absent, (1) present.
112 Septal nectaries or potentially homologous basal intercarpellary nectaries (0) absent, (1) present.
113 Number of ovules per carpel (0) one, (1) two or varying between one and two, (2) more than two.
114 Placentation (0) ventral, (1) laminar-diffuse or “dorsal.” Described as ventral by Friis et al. (2015) based on the relation of the ovule to the bract, but the bract shows the orientation of the carpel relative to the inflorescence axis, not to the floral axis, which is unknown. We scored living Chloranthaceae and Ceratophyllum as ventral, based on development (Endress & Doyle, 2009), but potentially related fossils as unknown.
115 Ovule direction (0) pendent, (1) horizontal, (2) ascendent.
116 Ovule curvature (0) anatropous (or nearly so), (1) orthotropous (including hemitropous).
117 Integuments (0) two, (1) one.
118 Outer integument shape (0) semiannular, (1) annular. Orthotropous taxa are scored as unknown.
119 Outer integument lobation (0) unlobed, (1) lobed.
120 Outer integument thickness (at middle of integument length) (0) two cells, (1) two and three to four, (2) four and five, or more; ordered. Friis et al. (2015, p. 199) stated that there may be only two cell layers, but that this is uncertain because it is difficult to distinguish the exotesta from the fruit wall and because there may be additional cells above holes in the endotesta (see Friis et al., 2015, fig. 14). Based on the latter, we score Canrightiopsis as 1.
121 Inner integument thickness (0) two cells, (1) two and three, or three, (2) three and more. Friis et al. (2015) described Canrightiopsis as having “several” layers of thin-walled cells, which are often collapsed, but their fig. 14 shows three cell layers; because we consider the state uncertain, we score Canrightiopsis as 1/2.
122 Chalaza (0) unextended, (1) pachychalazal, (2) perichalazal. Orthotropous taxa scored as unknown.
123 Nucellus (0) crassinucellar (including weakly so), (1) tenuinucellar or pseudocrassinucellar.
124 Fruit wall (0) wholly or partly fleshy, (1) dry.
125 Lignified endocarp (0) absent, (1) present. Scored only in fleshy fruits.
126 Fruit dehiscence (0) indehiscent or dehiscing irregularly, dorsally only, or laterally, (1) dehiscent ventrally or both ventrally and dorsally, (2) horizontally dehiscent with vertical extensions.
127 Hooked hairs on fruit (0) absent, (1) present.
128 Testa (0) slightly or non-multiplicative, (1) multiplicative. Because this character is defined by comparison with the number of cell layers in the ovule stage, it is not applicable in fossils.
129 Exotesta (0) unspecialized, (1) palisade or shorter sclerotic cells, (2) tabular, (3) longitudinally elongated, more or less lignified cells.
130 Mesotesta lignification (0) unlignified, (1) with sclerotic layer, (2) with fibrous layer. Characters 130 and 131 are scored as unknown (inapplicable) in taxa with a two-layered outer integument.
131 Mesotesta fleshiness (0) not juicy, (1) wholly or partly modified into a juicy sarcotesta.
132 Endotesta (0) unspecialized, (1) single layer of thin-walled cells with fibrous endoreticulum, (2) multiple layer of thin-walled cells with fibrous endoreticulum, (3) tracheidal, (4) palisade of thick-walled cells.
133 Tegmen (0) unspecialized, (1) thick-walled exo- and endotegmen, (2) fibrous to sclerotic exotegmen.
134 Ruminations (0) absent, (1) testal, (2) tegminal and/or chalazal.
135 Operculum (0) absent, (1) present.
136 Aril (0) absent, (1) present.
137 Female gametophyte (0) four-nucleate, (1) eight- or nine-nucleate.
138 Endosperm development (0) cellular, (1) nuclear, (2) helobial.
139 Endosperm in mature seed (0) present, (1) absent. Characters 139 and 141 are clearly visible with synchrotron radiation X-ray tomographic micrography.
140 Perisperm (0) absent, (1) from nucellar ground tissue, (2) from nucellar epidermis.
141 Embryo (0) minute (less than 1/2 length of seed interior), (1) large.
142 Cotyledons (0) two, (1) one.
143 Germination (0) epigeal, (1) hypogeal.
Rights and permissions
About this article
Cite this article
Doyle, J.A., Endress, P.K. Phylogenetic Analyses of Cretaceous Fossils Related to Chloranthaceae and their Evolutionary Implications. Bot. Rev. 84, 156–202 (2018). https://doi.org/10.1007/s12229-018-9197-6
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12229-018-9197-6