Abstract
Effective protection of endangered species is often limited by taxonomic discrepancies across state borders. This is also the case of the Dactylorhiza maculata agg. in Central Europe, where one to three species and several infraspecific taxa are recognized in various countries. Based on an extensive analysis of morphological variation, ploidy levels, environmental traits and habitats of 64 populations in Central Europe and adjacent regions, we aimed to propose a unified taxonomic concept applicable throughout the study area. Multivariate analysis of morphological traits revealed continuous variation at the individual level and only minor differences between particular clusters of populations. Four DNA-ploidy levels were detected using flow cytometry. Diploids (2n = 40) and tetraploids (2n = 80) were the most abundant and usually formed single-cytotype populations whereas DNA-triploids and DNA-hexaploids occurred only sporadically as minority cytotypes. The inferred patterns of morphological and ploidy variation were not congruent with traditional taxonomic treatment regarding diploid D. fuchsii and tetraploid D. maculata as two species with several infraspecific taxa. Instead, all taxa analysed in the current study are best treated at the subspecies level within D. maculata s. lat. due to somewhat continuous morphological variation between morphotypes. A total of eight D. maculata subspecies may be recognized in Central Europe, of which one is newly described here as D. maculata subsp. arcana, subsp. nov. Some nomenclatural riddles have been resolved, and the threat status of the recognized taxa is discussed.
Similar content being viewed by others
Introduction
The terrestrial orchid genus Dactylorhiza Neck. ex Nevski, distributed from the temperate to the boreal belt of the Northern Hemisphere with a centre of genetic diversity in the Mediterranean Basin and the Caucasus Mts, is one of the most taxonomically challenging groups of the orchid family (Pedersen 1998; Delforge 2006; Pillon et al. 2006; Eccarius 2016). With the exceptions of D. sambucina (L.) Soó and D. viridis (L.) R. M. Bateman, Pridgeon et M. W. Chase, all Central European members of the genus belong to the so-called D. incarnata / maculata polyploid complex. Within this complex, three groups can be recognized: the D. incarnata agg. (diploid only), the D. maculata agg. (comprising diploids and autopolyploids) and the D. majalis / traunsteineri complex, which includes allopolyploid derivatives of the previous two groups (Hedrén 2001; Pillon et al. 2007; Devos et al. 2005; Hedrén et al. 2008; Nordström and Hedrén 2009; Balao et al. 2016; Brandrud et al. 2020).
The evolutionary history and phylogeny of the D. maculata agg. has been explored using allozymes (Hedrén 1996), AFLP (Hedrén et al. 2001), nuclear and plastid markers (Hedrén 2003; Devos et al. 2003, 2005; Ståhlberg and Hedrén 2008, 2010; Naczk et al. 2015), and, most recently, RADseq data analyses (Brandrud et al. 2020). In general, all these methods revealed a similar pattern, dividing the D. maculata agg. into two major groups or clades, corresponding to two widely distributed taxa, namely D. *maculata and D. *fuchsii (the asterisk here and further on is used when dealing with taxa regardless of their taxonomic rank). The fuchsii group is considerably variable, but its genetic variation lacks any geographical structure. The maculata group, on the other hand, consists of two major evolutionary lineages with only a small contact zone between the southwestern and northeastern European lineage (Ståhlberg and Hedrén 2008, 2010). However, contradictory results have been obtained for some other taxa. For example, diploid D. *foliosa is either positioned as an early diverging group within the D. maculata agg. (Ståhlberg and Hedrén 2010), or it is nested within the maculata clade (Brandrud et al. 2020). The southeastern European diploid D. *saccifera is usually considered close to D. *fuchsii but may alternatively represent an early diverging clade of the whole group (Brandrud et al. 2020; Bateman 2021). Several other taxa with more regional distributions are sometimes included in large-scale phylogenetic studies, for example D. *caramulensis, D. *ericetorum, D. *islandica, D. *kolaënsis, D. *savogiensis or D. *transsilvanica, and they usually appear to be segregates of the maculata clade. However, because they are almost constantly under-represented, little is known about their genetic variation and phylogenetic position. Moreover, hybridization between members of particular groups / clades has been suggested to occur (e.g. Ståhlberg and Hedrén 2010; Naczk et al. 2015; Brandrud et al. 2020). The Madeiran endemic D. foliosa (Soland. ex Lowe) Soó is almost constantly recognized as a separate species, while the rest of the group may be treated as (i) a single species D. maculata (L.) Soó with three subspecies, namely subsp. maculata, subsp. fuchsii (Druce) Hyl. and subsp. saccifera (Brongn.) Diklić; (ii) two or more species, including D. maculata and D. fuchsii (Druce) Soó as the most frequent representatives; or (iii) a complex system of taxa recognized at the species, subspecies and variety levels.
These discrepancies are also apparent in the recent Central European taxonomic literature and regional floras with significant differences in the numbers of recognized taxa, their circumscription and, eventually, their taxonomic status (Table 1). A traditional concept of two species is applied in Hungary, where only D. maculata subsp. transsilvanica (Schur) Soó and D. fuchsii are recognized (Molnár and Csábi 2021), the latter alternatively including var. sooana ined. (Molnár 2011). A similar approach is applied in Germany (Müller et al. 2021), where a total of five taxa are recognized: D. maculata subsp. maculata, D. maculata subsp. elodes (Griseb) Soó, D. fuchsii subsp. fuchsii, D. fuchsii var. sudetica (Rchb.f.) H. Baumann, Künkele et R. Lorenz, and D. fuchsii subsp. psychrophila (Schltr.) Holub. However, the last has been recently rejected by Hassler and Muer (2022). Only D. maculata s. lat. is mentioned in the field guide to Austrian flora because of the unresolved taxonomy of the group (Fischer et al. 2008), but Redl (2003) recognized as many as three species in this country, namely D. maculata, D. sudetica (Rchb.f.) Averyanov, and D. fuchsii (incl. subsp. psychrophila). In Czechia, D. maculata is reported to consist of subsp. maculata, subsp. transsilvanica and subsp. elodes whereas D. fuchsii is divided into subsp. fuchsii, subsp. sooana ined. and subsp. psychrophila (Ponert 2019). The latter subspecies is treated at the species level by Mirek et al. (2020), who thus recognized a total of three species in Poland, D. maculata, D. fuchsii and D. psychrophila (Schltr.) Aver. The most intricate taxonomic concept is applied in Slovakia, where D. maculata, D. fuchsii and D. ericetorum (Linton) Aver. are recognized at the species level. Dactylorhiza maculata is further divided into three subspecies, namely subsp. maculata, subsp. transsilvanica and subsp. elodes, while D. fuchsii includes subsp. fuchsii and subsp. sooana (as ‘sooiana’; Vlčko et al. 2003).
The taxonomic concept used in a given country is mirrored in its national checklist, red lists, and legislation. It is thus crucial for the evaluation of the threat status of taxa recognized within any group (e.g. Bateman and Denholm 2003; Pillon et al. 2006; Joffard et al. 2022). A unification of these concepts across national borders, based on a thorough examination of the variation of the D. maculata agg., is therefore needed for the effective protection of its members at a European level. In this study, we analyse the morphological variability, cytotype diversity and habitat conditions of D. maculata agg. populations throughout Central European countries. Our aims for this study were to re-evaluate the morphological variation, cytotype diversity and ecological differentiation between particular taxa of this group. To this end, we have attempted to resolve some taxonomic and nomenclatorial ambiguities and to provide a unified taxonomic concept and determination key for the group that would be applicable throughout the study area. Finally, we assess the Red List categories of particular taxa in Czechia, for which thorough distribution data are available.
Material and Methods
Plant material and Designation of Taxonomic Groups
Data were sampled primarily in populations of D. maculata agg. in Central European countries (Austria, Czechia, Germany, Hungary, Poland and Slovakia). Additional populational samples were collected also in other parts of Europe, namely in Bulgaria, the Netherlands, Romania and Slovenia. For the purposes of the analyses detailed below, the populations were classified into several groups corresponding to taxonomic treatments used in the respective country (Vlčko et al. 2003; Molnár and Csábi 2021; Ponert 2019; Müller et al. 2021; Hassler and Muer 2022). Ambiguities were addressed as follows: (i) Because the taxonomic homogeneity of D. *elodes has been questioned (Vermeulen 1968; Sczepanski 2006; Kubát 2010), its populations from particular regions were analysed separately, distinguishing among elodes-WE (West Europe), elodes-BM (Bohemian Massif) and elodes-CA (Carpathians); (ii) A preliminary analysis of D. *transsilvanica (Taraška 2014) revealed a homogeneity of populations composed of typical plants and sympatric individuals with similar characters (morphological, karyological, ecological, and phenological), yet possessing flower and leaf pigmentation; all such plants were thus classified as D. *transsilvanica; (iii) Due to unsatisfactory treatment of the D. maculata agg. in Austrian and Polish literature, local populations were classified following the criteria used in neighbouring countries. In Poland, populations from the Bohemian Massif were determined following Ponert (2019), while those from the Carpathians and their foothills were classified according to Vlčko et al. (2003). In total, we recognized nine groups (Table 1): elodes-BM, elodes-CA, elodes-WE, ericetorum, fuchsii, maculata, psychrophila, sooana and transsilvanica. Several populations did not allow for unequivocal classification using the literature, so they were designated as ‘aggregate’ (also abbreviated as ‘agg’ in figures and tables). A total of 64 populations were used in the analyses; their list together with locality details is provided in Table S1 of the electronic supplementary material.
Morphometric Analysis
Morphological variability was assessed using univariate and multivariate morphometric analyses based on a total of 1,195 individuals originating from 58 populations (Table S1 in the electronic supplementary material), including 474 individuals from 25 populations of D. *fuchsii and D. *sooana used in a previous study (Taraška et al. 2021). The morphological characters under study included those that are traditionally used in determination keys and special taxonomic literature for the delimitation of various Dactylorhiza taxa as well as characters identified in our preliminary screening of Central European populations of the D. maculata agg. Altogether, 17 quantitative and 5 qualitative traits were measured or scored on living plants or on scans of flower lips; subsequently, 11 ratios were computed (Table 2; for a schematic illustration of the quantitative characters measured on examined plants, see Table S2a in the electronic supplementary material).
Six datasets were used for morphometric analyses. Pearson correlation coefficients were calculated for all datasets prior to all multivariate analyses to check for highly correlated pairs of quantitative characters (|r| ≥ 0.9). Whenever a pair of characters was highly correlated, one character from the pair was excluded. Multicollinearity in categorical characters was examined using Cramer's V (Legendre and Legendre 1998), but no pair of characters showed high association coefficients. An overview of the datasets, the types of OTUs used, groups and characters, and analyses performed is presented in Table 3.
Agglomerative hierarchical clustering (Ward’s and UPGMA methods) and principal component analysis (PCA), using Euclidean distance and standardization of traits to a zero mean and unit variance, were carried out using populations as operational taxonomic units (OTUs). The relative frequency of each state of particular categorical variable was considered as a quantitative variable. Principal coordinate analysis (PCoA) using Gower’s dissimilarity coefficient (Legendre and Legendre 1998) was used to obtain insight into the phenetic relationships among individuals of all groups studied and with the aggregate group excluded.
To test the morphological differentiation among a reduced set of seven groups and to identify the traits contributing the most to the differentiation among groups, partial least-squares discriminant analysis (PLS-DA; Barker and Rayens 2003; Scott and Crone 2021) was employed. The fuchsii and sooana groups, whose variability was previously studied by Taraška et al. (2021), were excluded from this reduced dataset in order to obtain more detailed insight into the variability of the other groups. Populations of the aggregate group were excluded as well, because they do not represent a coherent taxonomic unit. This reduced dataset was randomly divided into a training set (i.e. about 75% of the dataset) and a validation set (25%) balanced across the groups. Ten-fold cross-validation was used to estimate the number of components required for the best performance of PLS-DA. The area under the curve (AUC) was calculated from training cross-validation sets to complement the performance of PLS-DA and averaged across one-vs-all group comparisons. Using the final tuned model, variable importance in the projection (VIP), which is an indicator of the modelling power of a predictor in PLS, was calculated for each analysed morphological variable. Confusion matrices were constructed for the final model which summarizes the success of the reclassification / prediction of the observations for the training and validation samples, respectively.
To estimate whether a priori unclassified populations (the aggregate group) are really morphologically transient, they were passively projected into the ordination space in the PCA of populations, and an additional PCoA was carried out with all individuals as OTUs, including those of aggregate populations.
For each study group, descriptive data analysis was carried out to obtain basic statistics of quantitative traits and ratios (minimum, mean, maximum and standard deviation). For qualitative traits, the frequencies of particular states of character were calculated. To illustrate the variation in selected traits, box-and-whisker or stacked bar plots were used. The Kruskal–Wallis test was used for the comparison of quantitative characters and their ratios. Differences in qualitative characters were analysed by the χ2 test.
Most statistical analyses were performed using R 4.0.4 (R Core Team 2022). PCA and PLS-DA were computed using the mixOmics 3.15 package (Rohart et al. 2017) and the software xlstat (Addinsoft 2022), hierarchical clustering and descriptive statistics using the MorphoTools package (Koutecký 2015). PCoA was computed using Canoco 5.12 (ter Braak and Šmilauer 2012), ANOVAs, and log-linear models were run using the NCSS 9 software (NCSS 2013).
Ploidy Level Determination
DNA ploidy level was estimated by flow cytometry (FCM) following the protocol of Doležel et al. (2007). In total, 989 individuals from 64 populations were analysed (Table S1 in the electronic supplementary material). Plant material collected in the field was stored in a wet paper tissue at 4°C until processed, usually within 1–5 days. One or two ovaries of Dactylorhiza were analysed together with leaf tissue of the internal standard Pisum sativum cv. Ctirad (2C = 9.09 pg; Doležel et al. 1998). For triploids, the analysis was repeated with Zea mays cv. CE-777 (2C = 5.43 pg; Lysák and Doležel 1998). The nuclei solution was prepared by co-chopping the sample and standard tissue (Galbraith et al. 1983) in LB01 buffer with polyvinylpyrrolidone (PVP, 20 mg/ml; Doležel et al. 2007) in a Petri dish and subsequent filtration through a 40-μm nylon mesh. Before analysis, 30–50 μl of the respective fluorescent dye (depending on the laboratory and the type of flow cytometer) was added, which was either 4,6-diamidino-2-phenylindole (DAPI, 4 μg/ml) or propidium iodide (PI, 50 μg/ml). The samples stained with PI were also supplemented with 30 μl of RNase to digest RNA.
Four flow cytometers were used: BD Accuri C6 (BD Biosciences, San Jose, CA, USA) and Partec CyFlow ML (Partec GmbH, Münster, Germany) at the Department of Botany, Palacký University Olomouc; Partec CyFlow ML at the Department of Botany and Biodiversity Research, University of Vienna; and Partec CyFlow ML at the Institute of Experimental Botany, Olomouc. Individual plants were analysed as separate samples and the fluorescence of at least 3,000 particles was recorded in each run. FCM histograms were analysed in BD Accuri software or Partec FloMax software. Relative fluorescence was calculated for each plant as the ratio of the mean position of G0/G1 peak (cf. 2C-peak; Trávníček et al. 2015) of Dactylorhiza and the mean position of the G0/G1 peak of the internal standard. The ratios obtained from analyses with Z. mays were recalculated to P. sativum using a coefficient 2.25 (value obtained from several simultaneous measurements of Zea and Pisum). A subset of fourteen individuals were analysed with both fluorescent dyes (i.e. DAPI and PI) to assure compatibility between results obtained by different staining methods. These measurements were then used for the calculation of the ratio between DAPI and PI. The value of 0.88 was used to recalculate the standard:sample ratio of PI-stained samples. For the fuchsii and sooana groups, the same data were employed as in our previous study (Taraška et al. 2021).
Chromosome Counts
Gametophytic chromosome numbers (n) were established in immature pollinaria. Flower buds were collected ca 5–10 days before flowering, fixed in an ethanol : acetic acid (3 : 1) solution and stored at −20°C until use. The chromosomal spreads were made following the standard protocol of Feulgen staining (Weiss et al. 2003). Briefly, flower buds were hydrolysed in 5 N HCl for 30 min at room temperature, washed with water and stained with Schiff’s reagent (Sigma, Vienna, Austria) for 1–2 hours. Afterwards, pollinaria were extracted from the buds and squashed in 60% acetic acid. Chromosome spreads were observed under 1,000× magnification using an Olympus BX60 microscope equipped with an Olympus DP72 digital camera (both Olympus, Tokyo, Japan) and Axioplan light microscope (Carl Zeiss, Jena, Germany). Chromosomes were counted in at least ten cells per individual.
Environmental Differentiation Between Groups
To test associations of groups with environmental conditions, values for 19 bioclimatic variables and mean annual solar radiation, and 24 physical and chemical soil variables for each population were obtained from WorldClim 2.1 (Fick and Hijmans 2017) and SoilGrid 2.0 (Hengl et al. 2017), respectively. Bioclimatic and soil variables had a spatial resolution of ca 1 km and 250 m, respectively. Prior to the analyses, the variance inflation factor (VIF) was calculated for a set of variables and the highly correlated variables with biologically less meaningful importance were excluded from the set through a stepwise procedure using the ‘vifstep’ (th = 15) function from the usdm package (Naimi et al. 2014). Elevation as well as six bioclimatic and eight soil variables (from the top 5 cm soil layer) were preselected and analysed by discriminant analysis (DA) using Canoco 5.12. The significance of the first and all discriminant axes was evaluated by a Monte Carlo permutation test with 499 permutations. Additionally, the vegetation type of each population was recorded in the field and later reclassified into the phytosociological syntaxa using the level of phytosociological order according to the Hierarchical floristic classification system of European vegetation (Mucina et al. 2016). One habitat category was classified separately as forest roadside ditches because it was impossible to assign this habitat to any syntaxon. The frequency distribution of vegetation types for the groups studied was visualized as a mosaic plot. The aggregate group was excluded from the DA but included in the boxplot and mosaic plot.
Estimation of the IUCN Red List Categories
All members of the D. maculata agg. occurring in Czechia were evaluated against the Red List criteria following the methodology of IUCN (2012a, b). Data on their recent and former distribution were obtained from our current research, critically evaluated floristic records (Kaplan et al. 2017) and the Pladias database (Wild et al. 2019), with regard to differences in nomenclature and the circumscription of some taxa. The categories presented here substitute the categories previously published by Grulich (2017). The threat status was not estimated for other Central European countries because of a lack of data on geographic distribution and population abundance.
Results
Population-Level Morphometrics
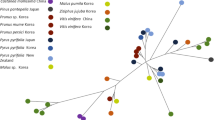
Cluster analysis of populations as OTUs (CLUST_1 analysis; Ward’s method; Table 3) resulted in two main clusters (‘a’ and ‘b’). Cluster ‘a’ included populations of the fuchsii and sooana groups, and cluster ‘b’ consisted of the rest of the groups (Fig. 1a). Using slice at a distance of 15, cluster analysis recognized seven clusters that mostly corresponded to the groups under study. The only exceptions were the elodes-BM and ericetorum groups and populations RUD and JES of the maculata group that were grouped together into one cluster, as well as population PBZ of the maculata group and SMU of the fuchsii group that were clustered with populations of the transsilvanica group (Fig. 1a). Cluster analysis using the UPGMA method (CLUST_2 analysis) also revealed clusters mostly corresponding to the groups studied using a smaller distance slice width (Table S3a in the electronic supplementary material), but the clustering pattern did not recognize two main clusters (‘a’, ‘b’) found by the CLUST_1 analysis (Fig. 1a).
Multivariate analyses of morphological traits of D. maculata agg. populations as OTUs. Groups are identified by different colours and symbols. a – Results of hierarchical cluster analysis using Ward’s method (CLUST_1 analysis, Table 3) with resulting clusters ‘a’ and ‘b’. Boxes demarcate clustered populations at the respective distance (d = 15). Codes of populations in bold and normal styles represent (predominantly) diploid and tetraploid populations, respectively. Symbols below some population codes denote their group identity. * – SMU population of the fuchsii group misclassified into the cluster predominated by the transsilvanica group. Population codes are explained in Table S1 in the electronic supplementary material. b – Sample plot of the first two axes (PCA1, PCA2) of the PCA (PCA_1 analysis, Table 3). Variation explained by each axis is within parentheses. Predominantly diploid and tetraploid populations of the fuchsii group are distinguished by different symbols. c – PCA correlation plot of analysed characters. Only variables whose correlations exceed |0.50| with at least one axis are displayed in the plot. Group abbreviations are explained in Table 1 and character abbreviations in Table 2.
The main gradient revealed by the first axis of the PCA (PCA_1, Fig. 1b) corresponded to the differentiation between the fuchsii, sooana and partially also transsilvanica groups on the right-hand side and all other groups on the left-hand side. Populations of the respective groups usually tended to occur in close proximity, but no apparent discontinuities between clusters of neighbouring groups were identifiable in the ordination diagram. Populations of the elodes-BM and ericetorum groups clumped together. The first PCA axis was positively correlated mainly with leaf width (wL1), plant height (hPI), the ratio of plant height to the length and number of leaves (hPl/IL1, hPl/IL2, hPl/nrL) and some flower size/shape traits (E, HH). It was negatively correlated mainly with some flower size traits and their ratios (B, AD, BBC) and leaf shape (IL1/wL1). The shape of the leaf apex (sLA) was mostly obtuse on the right and most acute on the left of the first PCA axis. The second PCA axis was mostly related to the pigmentation of vegetative and flower parts of the plants. Along the second PCA axis, the frequency of populations with a pink to purple labellum (cLBp) with bold markings (mLBb) and darker parts of inflorescence (ipInf) decreased, and the frequency of populations with a white labellum (cLBw) with absent markings (mLBa) increased (Fig. 1c). No morphological differentiation between diploid and tetraploid populations of the fuchsii group was identifiable from the PCA (Fig. 1b). Passively projected aggregate populations within the PCA diagram (PCA_2 analysis, Table S3b in the electronic supplementary material) filled the ordination space in-between several groups, namely the fuchsii, maculata, psychrophila and elodes-CA groups.
Because the relationships between populations within cluster ‘a’ have already been studied by us in another paper (Taraška et al. 2021), we conducted further multivariate analyses with populations of cluster ‘b’ (dataset 5; Table 3). The ordination space of the first three PCA ordination axes (PCA_3 analysis, Fig. 2a, c) showed the clustering of populations of each studied group, but the elodes-BM and ericetorum groups clustered together. Characters correlated with the first PCA axis indicated that plants of the elodes-WE group typically had a high spur length / width ratio (lSp/wSp), an acute leaf apex (sLA1a, sLA2a) and a narrow middle lobe of the lip (F/E). On the opposite side of the first PCA axis, plants of the transsilvanica group were typically taller (hPl), with subacute to obtuse apices of the leaves (sLA2s, sLA2o), and flowers often having a white labellum (cLBw) without markings (mLBa; Fig. 2b). The psychrophila populations strongly separated from the other groups along the second PCA axis (Fig. 2a), mainly due to intensive pigmentation of their lips (cLBd) as well as other parts of the inflorescence (ipInf), and several traits related to plant height and stature (Fig. 2b). The third PCA axis (Fig. 2c) separated populations of the elodes-WE group with the lowest scores and the elodes-CA group with the highest scores from the populations of other groups with intermediate scores. Plants of the elodes-WE group had flowers with a relatively short spur (lSp/A) and low Heslop-Harrison index (HH) and their leaves were widest in the basal part (lL2/mL2), while plants of the elodes-CA group had flowers with both absolutely and relatively long spur (lSp, lSp/A), and rather intensely pigmented both inflorescence (mLBb, ipInf) and leaves (spLb; Fig. 2d).
Multivariate analyses of morphological traits of D. maculata agg. populations as OTUs, with populations of the fuchsii and the sooana groups excluded (dataset 5, Table 3). Results of PCA_3 with (a, b) axes 1 and 2 and (c, d) axes 1 and 3, with sample plots and correlation plots. Variation explained by each axis is within parentheses. Only variables whose correlations exceed |0.50| with at least one axis are displayed in the plot. Group abbreviations are explained in Table 1 and character abbreviations in Table 2.
Individual-Level Morphometrics
The first two axes of the PCoA of individuals as OTUs (PCoA_1 analysis, Fig. 3a, Table 3) revealed an almost identical pattern as that found in the PCA of populations as OTUs (PCA_1 analysis, Fig. 1b) but with marked overlap among groups. While the first PCoA axis represented a composite gradient of both quantitative and qualitative characters, the second PCoA axis was primarily correlated with qualitative characters related to the colour of the labellum (cLB) and spots on the leaves (spL), separating plants with a white labellum (cLBw) with absent or pale markings (mLBa, mLBp) and leaves without spots (spLa) in the upper part from the plants with darker flowers (cLBp) and intesly pigmented inflorescences (ipInf) in the bottom part of the ordination diagram (Fig. 3b).
Multivariate analyses of morphological traits of D. maculata agg. individuals as OTUs, with populations of the aggregate group excluded (dataset 2a, Table 3). a – Sample plot of the first two axes (PCoA1, PCoA2) of PCoA_1 (Table 3). Variation explained by each axis is within parentheses. b – PCoA correlation plot of characters analysed. Only variables whose correlations exceed |0.40| with at least one axis are displayed in the plot. Group abbreviations are explained in Table 1 and character abbreviations in Table 2.
Plants of the aggregate group included in the PCoA (PCoA_2 analysis, Table 3) were spread over most parts of the ordination diagram, but most of them were placed in its bottom part, where they overlapped with marginal parts of the morphospaces of several other groups, namely the fuchsii, elodes-BM, ericetorum, elodes-CA, and psychrophila groups (Table S3c in the electronic supplementary material).
Univariate descriptive statistics are presented in Table S2b, c, d in the electronic supplementary material. Box-and-whisker plots or stacked bar plots of the traits studied for each group (DS_1 analysis; Table 3) are presented in Table S3f, g in the electronic supplementary material.
PLS Discriminant Analysis
PLS discriminant analysis of individuals (PLS-DA_1 analysis, Table 3) estimated the number of 8 predictive components to be optimal for the final model, with R2Xcum = 0.570, R2Ycum = 0.451, and Q2 cum = 0.394. This suggests a rather complex structure of the dataset. Seventeen variables (or their categories) had a VIP > 1 and could be considered important for discrimination between groups (Table S3d in the electronic supplementary material), with two qualitative (cLB, mLB) and four quantitative variables or ratios (lSp, wSp, lL1/wL1, lSp/A) having the highest VIP. The distribution of individuals of groups in the space of the first four components showed satisfactory discrimination of the transsilvanica group from the elodes-BM and the elodes-WE groups on the first component, and the psychrophila group vs. most other groups on the second component (Fig. 4a). Adding the third and fourth components differentiated the elodes-WE and the elodes-CA groups from most other groups (Fig. 4b, c). Only the maculata group was difficult to discriminate from the other groups, which is also clear from the cumulative AUC values (Table S3e in the electronic supplementary material) and the confusion matrices (Table 4). The analysis revealed that 81.6%/77.2% of the individuals could be correctly reclassified / predicted in the training / validation subsets. The maculata and ericetorum groups resulted in the lowest classification accuracy, approaching 51.9%/52.4% and 59.1%/38.9% (training / validation subset), respectively. The elodes-CA and elodes-BM groups showed an intermediate percentage of correctly classified individuals (73.7%/70.0%; 68.3%/68.8%). More than 95% of individuals in other groups were correctly reclassified / predicted in both training and validation subsets. The largest morphological overlap was found between the maculata and the transsilvanica groups and between the elodes-BM and the ericetorum groups (Table 4).
Sample plots and correlation plots from partial least squares discriminant analysis (PLS-DA_1) of individuals of seven taxonomic groups of D. maculata agg. (dataset 3, Table 3), divided into training (75% of dataset) and validation (25%) samples and balanced across the groups. The first four predictive components as axes are visualized. a – PLS-DA1 vs PLS-DA2, b – PLS-DA1 vs PLS-DA3, c – PLS-DA1 vs PLS-DA4. Ellipses are drawn for each group representing 95% quantile of the approximated bivariate normal density distribution. Only variables with Pearson correlations > |0.30| with at least one predictive component within each plot are displayed in the respective correlation plot. Variability of the Y matrix (intergroup variability) explained by respective predictive components (in %) are displayed within parentheses. Group abbreviations are explained in Table 1 and character abbreviations in Table 2. Large-sized symbols represent training samples, and small-sized symbols represent validation samples passively projected into the plots.
Chromosome Numbers and Ploidy Level Screening
Chromosome numbers were established for ten individuals representing five groups. Two different gametophytic chromosome counts were encountered among the plants analysed: n = 20 and n = 40, corresponding to diploids and tetraploids, respectively. Diploid chromosome numbers were found in the sooana group and one individual of the fuchsii group (see also Taraška et al. 2021), while tetraploid plants belonged to the elodes-CA, elodes-BM, elodes-WE and fuchsii groups. These counts were used to calibrate the results of the flow cytometry analyses (Table S4 in the electronic supplementary material).
Two major ploidy levels were found: diploids and tetraploids. Furthermore, two minority cytotypes were detected, for which chromosome numbers were not established, with relative fluorescence corresponding to DNA-triploids and DNA-hexaploids. Diploids were confined to the sooana group and about one-third of analysed individuals of the fuchsii group, while relative fluorescence corresponding to tetraploids was detected in some individuals of the fuchsii group, the majority of individuals of the elodes-CA and transsilvanica groups, and in all individuals of the elodes-BM, elodes-WE, maculata and psychrophila groups (Table 5). DNA-triploids were detected only in the fuchsii group, and DNA-hexaploids were found within the elodes-CA and transsilvanica groups (Table 5). These cytotypes always co-occurred in mixed-ploidy populations with some of the major cytotypes.
Environmental Differentiation Between Groups
Discriminant analysis of environmental variables produced eight discriminant axes (1. DA: pseudo-F = 0.3, P = 0.002, all DA: pseudo-F = 2.7, P = 0.002) and showed that the populations of the elodes-WE and psychrophila groups were the most distinct in terms of environmental conditions (Fig. 5a). Populations of the elodes-WE group were situated at the lowest elevations, having the lowest amount of solar radiation (Srad), the lowest values of temperature (Bio4) and precipitation seasonalities (Bio15), and the highest mean annual temperature (Bio1). Populations of the psychrophila group occupied the highest elevations above 1,100 m a.s.l., with the lowest mean annual temperature (Bio1) and isothermality (Bio3), high cation exchange capacity (CECSOL) and the highest soil organic matter content (ORCDRC) (Fig. 5a, Table S5 in the electronic supplementary material).
Sample plots and plots of relative importance of factors for group separation from discriminant analysis (DA) of environmental conditions extracted from the WorldClim and SoilGrid databases for the sites of groups of D. maculata agg. studied (abbreviations explained in Table 1). The first two components are visualized in each diagram. a – DA of nine groups with aggregate group excluded, b – DA of seven groups with the aggregate, elodes-WE and psychrophila groups excluded. The proportion of intergroup variability explained by the respective discriminant axis (in %) is displayed within parentheses. Explanations of variables (for details see Fick and Hijmans 2017; Hengl et al. 2017): Elevation – elevation; Srad – mean annual solar radiation; BIO1 – mean annual temperature; BIO3 – isothermality (BIO2/BIO7) (×100); BIO4 – temperature seasonality (standard deviation ×100); pH(KCl) – soil pH measured in KCl solution; pH(H2O) – soil pH measured in water solution; SLTPPT – weight percentage of the silt particles (0.0002–0.05 mm); CLYPPT – weight percentage of the clay particles (< 0.0002 mm); ORCDRC – soil organic carbon content; CECSOL – cation exchange capacity of soil; AWCh2 – available soil water capacity (volumetric fraction) with FC = pF 2.3; HISTPR – Histosols probability cumulative. Only the best discriminating variables are shown in the diagrams.
Discriminant analysis of the reduced dataset (without the elodes-WE and psychrophila groups) produced six discriminant axes (1. DA: pseudo-F = 0.3, P = 0.004, all DA: pseudo-F = 1.8, P = 0.006) and revealed that the populations of the sooana and transsilvanica groups and some populations of the fuchsii group situated on the right side of the diagram occupied sites with higher temperature seasonality (Bio4) and amount of solar radiation (Srad) and soils with higher pH and proportion of clay particles (CLYPPT), lower participation of soil organic matter (ORCDRC), lower probability of histosol occurrence (HISTPR) and smaller available soil water capacity (AWCh2) (Fig. 5b, Table S5 in the electronic supplementary material). Populations of the maculata, elodes-BM, ericetorum and elodes-CA groups were situated on the opposite side of the diagram, preferring sites with a lower amount of solar radiation (Srad) and temperature seasonality (Bio4), and with more acidic soils (pH) containing higher amounts of organic matter (ORCDRC) and available soil water capacity (AWCh2). Populations of the fuchsii group were intermediate in climatic and soil variables between the groups mentioned above. Boxplots of selected bioclimatic and soil variables for each group are available in Table S5 in the electronic supplementary material.
However, being aware of the different sample sizes between the study groups, it is possible to observe habitat differences between them (Fig. 6). The elodes-WE and psychrophila groups each inhabited one specific vegetation type, only recorded in these groups. On the other hand, populations of the fuchsii group inhabited the widest range of vegetation types, including semi-anthropogenic habitats (forest road ditches). Populations of the elodes-BM and ericetorum groups occupied a narrower but mutually similar spectrum of vegetation types (predominantly Caricetalia fuscae, Vaccinio uliginosi-Pinetalia sylvestris), differing from the rest. Mesic, subxerothermic and Nardus grasslands were important components of the vegetation harbouring members of the sooana and transilvanica groups, while these vegetation types were only rarely recorded in connection with some of the other groups.
Evaluation of the Red List categories in Czechia
All taxonomically recognized groups have been successfully evaluated against the Red List criteria at the national level in Czechia. Only the fuchsii group was deemed near-threatened (NT), while the other five groups met the criteria of being under some level of threat. Four groups, namely maculata, sooana, psychrophila and transsilvanica, were classified as endangered (EN). They are threatened mostly because of their fragmented occurrence, declining area of occupancy, number of locations, and both the extent and quality of their habitats (criterion B). The sooana and transsilvanica groups also evince a low and declining number of individuals (criterion C). The category of critically endangered (CR) was inferred for the elodes-BM group, which grows at a single locality (with a few subpopulations) in Czechia, and it is confined to vanishing habitats (criterion B). For details on the evaluation see Table 6.
Discussion
A high level of morphological variability was observed among Central European populations of D. maculata agg. They could be assigned to several morphotypes which were, however, weakly separated at both the individual and the population level. Diploids formed a coherent group but were morphologically indistinguishable from some tetraploids. Furthermore, the occasional occurrence of DNA-triploids and DNA-hexaploids pointed to recurrent polyploidization and/or hybridization between major cytotypes. Such a pattern challenges taxonomic concepts which recognize two or more distinct species within the D. maculata agg. in the study area. Despite that, a total of eight morphotypes with particular geographical, ecological or karyological attributes were inferred to exist and were circumscribed for Central Europe. These can be evaluated taxonomically.
Morphological Variability and Ploidy Level Diversity
Leaf morphology, lip shape and flower colouration are generally used for the delimitation of particular taxa within the D. maculata agg. (e.g. Vermeulen 1947; Heslop-Harrison 1951; Bateman and Denholm 1988; Dufrêne et al. 1991; Ståhlberg and Hedrén 2008), and they were also crucial in this study. The main gradient of morphological variability stretched from broad-leaved plants with a deeply three-lobed lip, corresponding to the fuchsii and sooana groups, to narrow-leaved plants with a nearly-entire lip, representing the elodes-WE, elodes-BM and ericetorum groups. Still, these extreme morphotypes were interconnected by the other groups (elodes-CA, maculata, psychrophila, transsilvanica). The other important gradient was related to flower pigmentation. This was crucial for the separation of the sooana from the fuchsii group, the elodes-CA and psychrophila groups from the maculata group, but also the transsilvanica group from the rest of the populations.
With the exceptions of the ericetorum and elodes-BM groups, each group represented a more or less coherent assemblage of populations, representing unique morphotypes. Populations of the ericetorum and elodes-BM groups formed a single coherent cluster, obviously assembling taxonomically identical plants, for which different names are used in various countries, specifically D. ericetorum in Slovakia (Vlčko et al. 2003) and D. maculata subsp. elodes in Czechia (Ponert 2019). Populations of the maculata group were morphologically coherent, but they alternately clustered with other groups, which stemmed from their intermediate morphological characteristics and difficult delimitation from other groups. Despite these ambiguities, the maculata group could not be unambiguously merged with any other group. Moreover, the unsatisfactory segregation of the maculata group from the elodes-CA, psychrophila and transsilvanica groups was likely to be caused by poor population sampling of these taxa, which reflects their overall rarity in the study area (cf. Vlčko et al. 2003; Kaplan et al. 2017).
Although it was usually possible to delimit individual groups in the analysis of populations, the analysis based on individuals revealed serious overlaps between pairs of morphologically similar groups, which points to fully continuous morphological variability within the D. maculata agg. (see also Naczk et al. 2015). Morphologically ambiguous individuals belonging to the D. maculata agg. are usually considered primary hybrids between particular taxa, most often D. *maculata and D. *fuchsii (e.g. Druce 1915; Heslop-Harrison 1948; Ståhlberg 2009). However, not only single individuals, but whole morphologically transitional populations occur in Central Europe, disrupting the discontinuities even at the population level. The overall variation of the D. maculata agg. in Central Europe thus seems to be more complicated than reported from Western and Northern Europe (e.g. Heslop-Harrison 1951; Tyteca and Gathoye 2003; Ståhlberg and Hedrén 2008).
The polyploid system of the D. maculata agg., too, is more complex than previously believed (e.g. Heslop-Harrison 1968; Vöth and Greilhuber 1980; Delforge 2006; Kubát 2010), as indicated by several studies (Jagiełło and Lankosz-Mróz 1988; Ståhlberg and Hedrén 2008, 2010). Four DNA-ploidy levels were detected in our FCM analysis, corresponding to diploids, DNA-triploids, tetraploids and DNA-hexaploids. Only diploids and tetraploids formed single-cytotype populations whereas DNA-triploids and DNA-hexaploids always occurred as minority cytotypes within mixed-ploidy populations. The frequency of polyploidization and ploidy level diversity within the D. maculata agg. thus resembles that of Gymnadenia conopsea (Trávníček et al. 2011, 2012), which is a representative of the phylogenetically closest genus (Bateman et al. 2003, 2018).
Diploid populations were strictly concentrated within the sooana and fuchsii groups whereas the other groups, including unclassified (aggregate) plants, comprised only tetraploids (with sporadic DNA-hexaploid individuals). Moreover, a considerable number of tetraploid individuals, morphologically indistinguishable from diploids, were found in the fuchsii group, which also assembled all DNA-triploids. Two processes may be involved in the formation of minority cytotypes: heteroploid hybridization and polyploidization via unreduced gamete formation (Kolář et al. 2017). Triploids are mostly regarded as hybrids between diploid and tetraploid individuals of the genus Dactylorhiza (e.g. Heslop-Harrison 1968; Lord and Richards 1977; Pedersen 2006; Ståhlberg 2009), which is also a common way of triploid formation in vascular plants (cf. Popelka et al. 2019; Koutecký et al. 2022). Hexaploids are more likely to originate as a result of unreduced gamete formation within tetraploid populations (Ståhlberg and Hedrén 2008), which may also be the case with some triploids found in diploid populations (cf. Kobrlová et al. 2022; Gajdošová et al. 2023; Vojtěchová et al. 2023).
The evolutionary and taxonomic significance of ploidy level variation within the D. maculata agg. has been a matter of dispute. Differences in chromosome numbers have long been held to represent a strong reproductive barrier and a good predictor of morphological characters in Northern and Western Europe (e.g. Heslop-Harrison 1951; Tyteca and Gathoye 2003). Also in Central Europe, the ploidy level has traditionally been believed to be the most important character distinguishing between D. fuchsii (diploid) and D. maculata (tetraploid), despite their morphological similarity (e.g. Borsos 1961; Vöth 1978; Procházka 1979; Kubát 2010). Nonetheless, reproductive barriers between cytotypes are sometimes bypassed, resulting in gene flow across ploidy levels (Hülber et al. 2015; Kolář et al. 2017; Hanušová et al. 2019). The tetraploidy of Central European populations of D. *fuchsii may further facilitate its hybridization with other taxa of the group (Ståhlberg and Hedrén 2010; Naczk et al. 2015; Brandrud et al. 2020). This might have led not only to the establishment of primary hybrids between distinct tetraploid lineages, but also to the origin of morphologically transitional populations (here referred to as the aggregate group). This hypothesis should be tested further by molecular methods focused on population genetics.
Habitat and Environmental Differentiation Among Groups
Diploids and tetraploids of the D. maculata agg. have been reported to occupy different (micro)habitats, mainly depending on light conditions and soil pH (Heslop-Harrison 1951; Vaucher 1966; Dufrêne et al. 1991; Tyteca and Gathoye 2003; Ståhlberg 2009), which was sometimes thought to support their separation into two species, namely D. fuchsii growing in more shaded (forest) habitats on base-rich soils and D. maculata found in open peat bogs and meadows on acidic soils (e.g. Heslop-Harrison 1951). However, our analysis revealed a more complex pattern. We partially confirmed the observations of Jagiełło (1988) that there is a correlation between leaf shape and soil pH, as some narrow-leaved groups (e.g. the elodes-BM, elodes-WE groups) were associated with extremely acidic soils whereas groups characterized by broad leaves (e.g. the fuchsii, sooana groups) were found on just slightly acidic soils. However, the rather narrow-leaved transsilvanica and broad-leaved sooana groups had almost the same soil pH requirements and shared some habitat types. In addition, the environmental niche of the sooana group was clearly distinct from that of the fuchsii group despite their morphological similarity. Furthermore, the fuchsii group, regardless of its ploidy level, was found to grow in a wide range of habitats, including woodlands, forests and meadows, with different environmental conditions, for example soils with a wide range of pH. Such a diversity of habitats occupied by D. *fuchsii has also been reported by Kirillova et al. (2022) from the Ural Mts.
Consistently with the general ecological pattern of niche breadth and geographic range size (Slatyer et al. 2013), groups with larger distribution areas, such as fuchsii or transsilvanica, occupied a wider range of habitats and tolerated more diverse environmental conditions whereas groups with local distributions (e.g. elodes-CA, elodes-BM, ericetorum, psychrophila) were usually confined to specific habitats (e.g. open coniferous woods in oligotrophic mires, subalpine water-springs) that have a very sparse, patchy distribution pattern across Central Europe. The morphological distinctions of the latter groups may thus be partly explained by the habitat-island effect (Mendez-Castro et al. 2021) and the gradual morphological and ecological differentiation of isolated populations (cf. Majeský et al. 2022). In addition, also quaternary climatic oscillations (Roy et al. 1996) may have facilitated contacts between distinct lineages, resulting in the establishment of locally distributed hybridogeneous populations that later became ecologically and geographically isolated from their parents (Kadereit 2015).
It remains unclear to what extent morphology can be affected by the environment and whether some local morphotypes do not in fact represent ecotypes rather than taxa (cf. Lowry 2012). On the other hand, environment-induced adaptive changes in Dactylorhiza may be stabilized by epigenetic changes, which are hardly detectable even by conventional molecular methods but enable the ecological separation of taxa with similar genomes (Paun et al. 2011). Our observations suggest that the environment may shape individual phenotypes only to some extent and that similar habitats can be occupied by different morphotypes, which may be obviously attributed to different (epi)genotypes. For example, both the elodes-BM and maculata groups can colonize transitional mires; the elodes-CA and transsilvanica groups can colonize fen meadows; the fuchsii and sooana groups can colonize beech woodlands or forests, etc. However, the resolution of our environmental data is rather coarse and these limitations must be taken into account when interpreting environmental differences between the groups. Whereas our soil data have a spatial resolution of 250 m, habitat differentiation between distinct cytotypes may be apparent at much finer spatial scales (Ståhlberg 2009; Šafářová and Duchoslav 2010).
An Intricate Pattern of Morphological, Cytogenetic and Ecological Variability Supports the Concept of a Single Species
A total of four distinct groups were recognized in a recent phylogenetic study among European D. maculata agg. taxa (Brandrud et al. 2020): D. *saccifera clade, D. *gervasiana clade, D. *fuchsii clade and the substantially heterogeneous D. *maculata clade, which included representatives of several taxa, among others D. *foliosa and D. *transsilvanica, but also plants termed as D. *ericetorum. However, their topology (reviewed by Bateman 2021) was unstable and with low bootstrap values, especially with respect to the D. *fuchsii and D. *maculata clades. Moreover, some taxa (e.g. D. *fuchsii and D. *transsilvanica) were rather undersampled regarding their variability and geographical distribution area. Despite these ambiguities, Bateman (2021) argued for a taxonomic concept treating the four clades resolved by Brandrud et al. (2020) as separate species. Still, however, he allowed for the Madeiran endemic D. foliosa to be recognized at the species level because of its morphological divergence from D. maculata s. str., which was thus rendered paraphyletic. An alternative taxonomic concept which complies with the phylogeny elucidated by Brandrud et al. (2020) is considering the whole D. maculata agg. as one species with multiple infraspecific taxa, typically subspecies (Baumann et al. 2002; Ströhle 2003; Conti et al. 2005; Ståhlberg and Hedrén 2010; Naczk et al. 2015; Průša 2019; Taraška et al. 2021). This rather conservative treatment was rejected by Bateman (2021) because it lacks a hierarchical framework of classification.
The most discussed ambiguities in D. maculata agg. relate to the delimitation of D. maculata s. str. and D. *fuchsii. In Western and Northern Europe, they seem to be well distinguishable based on morphology and ploidy level (e.g. Heslop-Harrison 1951; Bateman and Denholm 2003; Tyteca and Gathoye 2003; Ståhlberg and Hedrén 2008). The traits used for discrimination, however, often fail in Central Europe, where tetraploids of both taxa occur and boundaries between them are weakened by reciprocal gene flow (Ståhlberg and Hedrén 2010; Naczk et al. 2015; Brandrud et al. 2020). This was also apparent in our data. Clustering using Ward’s method was found to be the most congruent with classifications based on molecular data (e.g. Ståhlberg and Hedrén 2010; Bateman 2021), dividing the dataset into two main clusters corresponding to D. *fuchsii clade and D. *maculata clade as recognized by Brandrud et al. (2020). However, other methods did not show such a clear pattern, as the clustering of groups was highly unstable. In other words, some groups could not be unequivocally subordinated either to D. *maculata or D. *fuchsii. Previously, this was manifested by the unstable taxonomic treatment of taxa represented by these groups. For example, populations of the psychrophila group have been alternately incorporated into D. maculata (Jagiełło 1988; Eccarius 2016) or D. fuchsii (Baumann et al. 2004; Kreutz 2004; Kubát 2010), or set aside as a separate species (Redl 2003; Delforge 2006; Mirek et al. 2020; see also Table 1). Serious difficulties have also been reported with regard to distinguishing between D. *fuchsii and D. *transsilvanica, traditionally subordinated to D. *maculata (cf. Borsos 1961; Bernátová et al. 1993; Baumann et al. 2002; Kubát 2010), but sometimes also to D. *fuchsii (e.g. Baumann et al. 2004; Jäger and Werner 2006). After all, misidentifications and confusions are frequent even between D. *fuchsii and D. *maculata (Kaplan et al. 2017). Unlike in Atlantic and Nordic Europe, where D. *maculata is reported to be clearly distinct from other taxa, it occupies a central position within the overall, more or less continuous, morphological variability of the D. maculata agg. in Central Europe. In this area, it may be considered a transitional morphotype between broad-leaved fuchsii and narrow-leaved groups of ericetorum and elodes-BM. It is also morphologically close to the elodes-CA, psychrophila and transsilvanica groups, which, however, differ by a set of quantitative and, above all, qualitative traits.
The observed patterns of morphological variability, cytotype diversity and eco-sociological attributes do not allow for a hierarchical classification of the D. maculata agg., which is here treated as a single species – D. maculata. Some of its Central European members with a limited distribution area and distinctive morphological and ecological properties may be derived from widely distributed lineages of the D. *maculata clade and the D. *fuchsii clade, which would make them analogous to D. *foliosa in Brandrud et al. (2020). By contrast, some other taxa are likely to represent introgressions between these two clades, particularly the psychrophila and transsilvanica groups. Moreover, transitional populations (here referred to as the aggregate group) were recorded between the fuchsii / maculata (53, Suché kopce; 61, Zinnwald), fuchsii / psychrophila (55, Velká kotlina), ericetorum / maculata (35, Pavlová) or even fuchsii / maculata / psychrophila (17, Horská louka u Háje) groups.
Therefore, the rank of subspecies seems to be most appropriate for all these taxa. It is also congruent with the taxonomic treatment applied to the allopolyploid taxa of the D. majalis / traunsteineri complex subordinated to the species D. majalis despite their multiple origins (Bateman and Denholm 1983; Pedersen et al. 2003; Nordström and Hedrén 2008, 2009).
Overview of D. maculata subspecies in Central Europe
Analysis of taxonomic concepts used in the regional literature (see Table 1) revealed that the circumscription of some taxa needed to be re-evaluated. Thus, a total of eight taxa may be recognized in the region (Fig. 7).
The fuchsii group represents D. maculata subsp. fuchsii (Druce) Hyl. (Fig. 8), the most widespread taxon of the D. maculata agg. in Central Europe. It is generally considered morphologically, karyologically and ecologically distinct from D. maculata s. str. and all its subordinated taxa. The morphological distinctiveness of the fuchsii group was partially observed also in our data, despite overlaps with other groups, mainly the transsilvanica and sooana groups. The separation of the fuchsii group became less clear after adding some unclassifiable tetraploid populations to the dataset, representing morphological transitions to the maculata or psychrophila groups (see above). It may be hypothesized that morphologically transitional populations arose from repeated hybridization between various tetraploid taxa, including D. *fuchsii. Simultaneously, gene flow between diploids and tetraploids of D. *fuchsii can be facilitated by recurrent polyploidization (Taraška et al. 2021). High genetic variation (Ståhlberg and Hedrén 2010; Naczk et al. 2015) may allow D. *fuchsii to grow in a number of environmental conditions and range of habitats, which results in relatively frequent co-occurrence with other taxa of the group. Thus, D. *fuchsii is likely to be involved in gene exchange with other taxa of the D. maculata agg. and it seems inappropriate to treat it as a separate species.
The sooana group has been identified as D. maculata subsp. sooana Batoušek, Taraška et Trávn. (Fig. 9). This taxon was first recognized by Borsos (1959) and validly described by Taraška et al. (2021). It is confirmed to occur only in the West Carpathians, with one plausible report on its occurrence in Transcarpathian Ukraine (Loya 2015); records from other areas are likely misidentifications. It is a regional vicariant of D. maculata subsp. fuchsii, from which it differs in having a strictly diploid chromosome number and a distinct pattern of pigmentation, always having white anther caps and, simultaneously, spotted leaves, but also in its occurrence in more mesic and thermophilous habitats. A detailed analysis of this taxon and its relations to D. maculata subsp. fuchsii has been provided elsewhere (Taraška et al. 2021).
Various taxa used to be recognized as D. *elodes in different European regions (Vermeulen 1968; Sczepanski 2006). Three geographically distinct groups of this taxon were therefore established for the purpose of our analysis, namely elodes-WE, elodes-BM and elodes-CA. Dactylorhiza maculata subsp. elodes (Griseb.) Soó was described by Grisebach (1845) as Orchis elodes Griseb. from the Atlantic wet heaths in the border area of Germany and Netherlands. This name should therefore be primarily applied to populations represented by the elodes-WE group in our study (Fig. 10). They were clearly morphologically separated from all other groups in our analysis, including the elodes-BM and elodes-CA groups. Also, the environmental conditions differ between the stands of the elodes-WE populations and populations from Central Europe. Moreover, differences were also found between both Central European elodes groups. Populations of the elodes-BM group appeared to be morphologically indistinguishable from those of the ericetorum group, which allowed us to amalgamate these two groups into one. By contrast, populations of the elodes-CA group were morphologically close to the maculata group, from which they differed by the number of stem leaves, the shape of the leaves, darker flowers, and flower lips with a more robust spur (Fig. 11). Because of these characters, the elodes-CA group may to some extent resemble plants of the D. majalis / traunsteineri complex, especially D. traunsteineri s. str. Other morphological traits as well as genome size integrate the elodes-CA group into the D. maculata agg., but introgression from other taxa cannot be ruled out. Moreover, populations of the elodes-CA group could not be reliably merged with any other group nor any taxon recognized in the area, and a new name D. maculata subsp. arcana, subsp. nov. is therefore proposed here (see below).
Populations assigned to the ericetorum and elodes-BM groups (Fig. 12) were characterized by extremely narrow leaves, up to 10–14× longer than wide, they represented a distinctive morphotype among all Central European plants, and they also typically occupied a specific habitat, namely open coniferous forests on mires. In Czechia, they are called D. maculata subsp. elodes (Ponert 2019), but this name should be applied to a different taxon (see above). The names based on the basionym Orchis maculata subsp. ericetorum E. F. Linton do not seem to be appropriate either. Linton (1900) characterized O. *ericetorum as plants with narrower leaves compared to typical ‘O. maculata’, but he misapplied the latter name to D. *fuchsii, which has relatively broader leaves (Vermeulen 1947; Sczepanski 2006). Vermeulen (1968) regarded O. *ericetorum as a variety of D. maculata (= D. maculata subsp. maculata) growing on heaths, and the names based on the epithet ‘ericetorum’ are also regarded as synonyms of D. maculata subsp. maculata in most of recent works (e.g. Bateman and Denholm 2003; Eccarius 2016). Anyway, the elodes-BM group also contained the population from the locus classicus of D. maculata subsp. averyanovii Jagiełło, described by Jagiełło (1990) from Zieleniec, Poland (loc. 60). This seems to be the only valid name for plants of the elodes-BM and ericetorum groups. Whether it applies also to the West European narrow-leaved populations, sometimes referred to as D. *ericetorum, must be scrutinized further.
The transsilvanica group corresponds to D. maculata subsp. transsilvanica (Schur) Soó (Fig. 13), which was described as Orchis transsilvanica by Schur (1853) and typified by his collection from Romania (Klein and Deutsch 2005). Plants from Slovenia and Bulgaria were reported to be tetraploids (Klein and Deutsch 2005; Petrova et al. 2009), but the ploidy level of plants in other parts of the subspecies’ distribution range was long uncertain (e.g. Kubát 2010). Our data confirmed tetraploidy in all studied populations, but one DNA-hexaploid plant was found in Slovenia. Dactylorhiza *transsilvanica is usually characterized by white flowers and unspotted leaves (e.g. Soó 1980; Delforge 2006; Eccarius 2016), which corresponds with the original description (Schur 1853). Sympatrically growing plants with different patterns of pigmentation, but the same morphological, karyological and habitat attributes, were usually determined as different taxa, typically D. *maculata or D. *fuchsii. However, such individuals were observed in all visited localities in Transylvania, that is, in the broad area classica. The situation at the type locality is unknown, as it has probably ceased to exist (V. Taraška and B. Trávníček, pers. observ.). These variable populations seem to be common in the Carpathians whereas populations of almost exclusively ‘pure’ (i.e. non-pigmented) D. *transsilvanica plants were only found in certain parts of its distribution range (Bílé Karpaty Mts, Dinarides and Stara Planina Mts). Such a pattern is analogous to that observed in D. sambucina with two flower-colour morphs, intermediate individuals and rarely occurring ‘pure’ populations of uniform flower colouration (Gigord et al. 2001; Jersáková et al. 2006). The generally accepted circumscription of D. *transsilvanica therefore needs to be extended so that it includes both its colour morphs and transitional individuals.
The psychrophila group aggregated populations of dwarf plants growing in subalpine habitats, usually recognized as D. fuchsii subsp. / var. psychrophila (e.g. Procházka 1979; Kubát 2010; Ponert 2019). Dactylorhiza *psychrophila was described by Schlechter (in Keller and Schlechter 1928:183) as ‘Orchis maculata var. psychrophila’, and its neotype comes from Lapland (Vermeulen 1947). Some authors (e.g. Averyanov 1990; Devillers and Devillers-Terschuren 2000; Baumann et al. 2002; Tyteca and Gathoye 2003; Delforge 2006; Eccarius 2016) suppose it to occur only in North Europe and Siberia, while several others consider it as an arctic–alpine taxon distributed also in Central European mountains (e.g. Soó 1980). In that area, taxonomic ambiguities stem from unresolved relations between D. *psychrophila and D. *sudetica. The latter was described as ‘Orchis maculata var. sudetica’ by Reichenbach (1850) based on plant material collected by Poech at an unspecified locality in the Sudeten Mts (Eccarius 2016), almost certainly in the Krkonoše Mts (cf. Klášterský et al. 1982). Both taxa are characterized by a subtle habitus and their affinity to similar habitats. Anyway, several distinctions have been identified between plants from Northern Europe and those from the Sudeten Mts. Nordic D. *psychrophila is usually deemed to be diploid (e.g. Averyanov 1990; Eccarius 2016), but plants from the Krkonoše Mts were found to be tetraploid (Jagiełło and Lankosz-Mróz 1988; Krahulcová 2003), which was also confirmed by our FCM screening. Furthermore, D. *psychrophila is considered morphologically close to D. *fuchsii (e.g. Averyanov 1983; Eccarius 2016), but Jagiełło (1988) pointed out the similarity of Central European populations to D. *maculata rather than D. *fuchsii. Also, populations in the Krkonoše Mts either clustered with the maculata group in our morphometric analysis or occupied an intermediate position between the groups of maculata and fuchsii. These circumstances justify the separation of plants from the Krkonoše Mts as distinct from Nordic D. *psychrophila as well as from all other Central European members of the D. maculata agg. Consequently, they should be recognized as D. maculata subsp. sudetica (Poech ex Rchb.f.) Vöth (cf. Jagiełło 1988; Delforge 2006; Eccarius 2016). Several populations in the Hrubý Jeseník Mts (55, Velká kotlina) and Krušné hory Mts (e.g. 17, Horská louka u Háje) are sometimes considered taxonomically identical to those from the Krkonoše Mts (e.g. Vlačiha and Dundr 2002; Kubát 2010; Bureš 2013; Kaplan et al. 2017), but this was not unequivocally confirmed in our analysis, and these populations thus remained unclassified. Müller et al. (2021) mentioned D. fuchsii var. sudetica from the Erzgebirge/Krušné hory Mts, but the same plants had been previously called D. *transsilvanica (Jäger and Werner 2006), and their taxonomic identity is unclear. The occurrence of plants morphologically similar to D. *sudetica in the Alps (e.g. Hassler and Muer 2022) is likely to be a result of parallelism in alpine habitats (Knotek et al. 2020; Španiel et al. 2023). According to the current state of knowledge, D. maculata subsp. sudetica (Fig. 14) should be regarded as an endemic of the Krkonoše Mts.
The maculata group did not possess any clearly distinctive characters, so it was the least structured group. Dactylorhiza maculata L. was described by Linné (1753:942) as Orchis maculata L. in merely a general manner covering virtually all taxa of the D. maculata agg. A lectotype was therefore selected by Vermeulen (1947). In the narrow sense, this name applies to the tetraploid taxon, which is quite common in Atlantic and Boreal parts of Europe (e.g. Hansson 1985; Dusak and Prat 2010) but rare in the rest of its distribution area spanning from Europe to Central Siberia (Eccarius 2016). It is reported from all Central European countries, but literature records are strongly biased by varying species circumscriptions and taxonomic concepts used by different authors (Kaplan et al. 2017). Only populations strictly corresponding to D. maculata s. str. were assigned by us to the maculata group (Fig. 15). Yet, some populations with less matching morphological characteristics should be probably included as well, particularly those in the Krušné hory Mts, where the occurrence of the south-west lineage of D. *maculata was also confirmed by molecular genetics (Ståhlberg and Hedrén 2010). Some of the local populations were treated as unclassified (the aggregate group) in our analysis, and their addition to the maculata group led to an even worse ability to discriminate between the maculata and other groups, mainly the fuchsii and psychrophila groups. On the other hand, the admittedly low number of maculata populations included in the analysis due to strict classification criteria may have contributed to the limited success of the statistical methods at distinguishing this group from all others. Still, D. maculata subsp. maculata must be regarded as the most average morphotype of the D. maculata agg., further challenging the traditional taxonomic concepts with two or more recognized species.
Checklist of recognized subspecies of D. maculata
Dactylorhiza maculata subsp. arcana Trávn., Taraška, Batoušek et Lamla, subsp. nov.
– D. maculata subsp. elodes auct. non (Griseb.) Soó 1962: Vlčko et al., Orchids of Slovakia 31 (2003)
Holotype: Polsko [Poland]: Tatry Zachodnie Mts, Kościelisko village (near Zakopane town), peat bog west of Biały Potok settlement, west of the village – 905 m.a.s.l.; 49° 16′ 59″ N, 19° 50′ 45″ E (WGS-84); 25 June 2016, leg. excursion group; OL 44443! (Table S6 in the electronic supplementary material).
Isotypes: OL 44441!, BRNM 840763!
Description: Perennial herbs with palmate tubers. Plants (26–)27–49(–67) cm high, with (4–)5–7(–8) sheathing leaves and 1–4(–5) bract-like leaves. Sheathing leaves narrowly oblanceolate, usually with bold or pale spots, sometimes unspotted, making an angle of ~ 30° with the stem; bract-like leaves smaller, lanceolate. The lowermost well-developed leaf (50–)70–141(–174) mm long and (10–)11–21(–29) mm wide, (1.6–)3.4–8.6(–10.5)× longer than wide, usually subacute at the apex. The 2nd lowermost leaf (80–)97–174(–219) mm long and (9–)11–21(–31) mm wide, (0.7–)5.9–11.5(–13.3)× longer than wide, with the widest dimension in its upper half, usually acute at the apex. Inflorescence a sparse to dense-flowered spike, often with dark reddish-purple anthocyanin pigmentation of the stem, bracts and/or ovaries. Tepals purple, often with bold markings. Lip three-lobed with rather small median lobe, pink to reddish-purple, nearly always with bold markings, the Heslop-Harrison index (1.0–)1.1–1.4(–1.5); spur robust, (7.4–)8.0–10.9(–12.3) mm long and (1.5–)1.9–2.9(–3.3) mm wide in the middle of its length, down-curved, darkly purple; flower colouration and spur shape somewhat resembling that of D. traunsteineri. Fruit a capsule with dust-like seeds.
Similar taxa: Dactylorhiza maculata subsp. arcana is similar to the type subspecies but differs in having dark (reddish-)purple flowers with robust spurs and narrower leaves, which are subacute at the apex and widest in their upper half. The two taxa also differ in several habitus-related traits, as individuals of D. maculata subsp. arcana more often have a densely foliated stem, more erect leaves and sparser inflorescences. It may be also confused with plants of the D. majalis / traunsteinerii complex, from which it differs in having a ‘maculata-like’ lip shape and genome size.
Chromosome counts and ploidy level: 2n = 4x = 80; exceptionally 2n ~ 6x.
Habitat and ecology: Moderately calcium-rich sedge-moss fens.
Phytosociological relevé: Poland, Kościelisko-Biały Potok, peat bog 920 m SSW from the confluence of the Kirowa Woda river and Lejowy Potok stream, GPS (WGS-84): 49° 16′ 59.8″ N, 19° 50′ 45.7″ E, ca 900 m.a.s.l., decl. 2°, exp. NW, area: 5 × 5 m; 28 June 2021, recorded by V. Taraška, P. Batoušek, F. Lamla and B. Trávníček; taxonomic nomenclature after Kaplan et al. (2019).
Cover – total: 99%; E3: 0%; E2: 1%, E1: 80%, E0: 99%. – E2: Salix aurita +, Salix caprea r, Salix pentandra r. – E1: Vaccinium oxycoccos 3, Carex panicea 2b, Eriophorum angustifolium 2b, Menyanthes trifoliata 2m, Potentilla erecta 2m, Carex dioica 1, Carex flava 1, Dactylorhiza maculata subsp. arcana 1, Drosera rotundifolia 1, Equisetum palustre 1, Pedicularis palustris 1, Trientalis europaea 1, Angelica sylvestris +, Calluna vulgaris +, Carex echinata +, Carex nigra +, Crepis paludosa +, Polygala vulgaris +, Briza media r, Carex rostrata r, Equisetum fluviatile r, Eriophorum vaginatum r, Festuca rubra r, Galium palustre r, Picea abies juv. r. – E0: Sphagnum spp., indet. – Species outside the relevé: Calla palustris, Eriophorum latifolium, Juncus squarrosus, Tofieldia calyculata.
Threat status: The subspecies should be considered critically endangered [CR B2ab(iii)] because of its rarity in both countries, Slovakia and Poland, at least until comprehensive data on its total distribution and population dynamics is gained.
Etymology: From the Latin word arcanus = mysterious, enigmatic. We suggest the epithet ‘tajomný’ for the Slovak and ‘tajemnicza’ for the Polish vernacular subspecies name.
Distribution: Endemic to Poland and Slovakia, with localities known in the foothills of the Oravské Beskydy Mts and Tatry Mts.
Dactylorhiza maculata subsp. averyanovii Jagiełło,
Acta Univ. Wratislav. 1055: 50 (1990)
≡ D. maculata subsp. elodes var. averyanovii Jagiełło, Fragm. Florist. Geobot. 31–32(3–4): 369 (1988)
– D. ericetorum auct. non (Linton) Aver. 1982: Vlčko et al., Orchids of Slovakia 25 (2003)
– D. maculata subsp. elodes auct. non (Griseb.) Soó 1962: Ponert in Kaplan et al., Key to the Flora of the Czech Republic 185 (2019)
Type (holotype): ‘Zieleniec (Sudeti Orientales, regio urbis Kłodzko), in margine sphagneti’, June 1982, M. Jagiełło, KRAM 297001 (digital image!).
Morphology: Relatively narrow linear leaves with parallel margins and acute apices, up to 19-(23)× longer than wide, avg. Heslop-Harrison index: 1.2.
Chromosome counts and ploidy level: 2n = 4x = 80.
Habitat and ecology: Open pine and spruce woods in oligotrophic mires, peat bogs and sedge-moss vegetation.
Distribution: Czechia, Poland, Slovakia. The Central and East Sudeten Mts, Beskydy Mts.
Threat status: Czechia: CR B1ab(iii)+2ab(iii). Slovakia: CR; evaluated as D. ericetorum (Eliáš et al. 2015). Poland: not evaluated (cf. Zarzycki and Szeląg 2006).
Taxonomic note: This taxon was initially treated at the subspecies level by Jagiełło, who later changed her opinion and lowered it to the rank of variety (cf. Jagiełło 1988, 1990). Because of a long delay in the publication of the first manuscript written, the subspecies name was unintentionally published later (Jagiełło 1990) than the varietal one (Jagiełło 1988). Nonetheless, both publications include literally the same description and refer to the same type specimen. Both names are therefore validly published, they are legitimate, and neither of them should be regarded as a basionym for the other; instead, they must be considered homotypic synonyms.
Dactylorhiza maculata subsp. elodes (Griseb.) Soó,
Nom. Nov. Generis Dactylorhiza 7 (1962)
≡ Orchis elodes Griseb., Goett. Studien: 276–277 (1845)
≡ Dactylorhiza elodes (Griseb.) Aver., Bot. Zhurn. 67(3): 309 (1982)
Type (holotype): ‘[Germany/Netherlands] Bourtangermoor’, sine dato, A. H. R. Grisebach (not signed), GOET 7217 (digital image!).
Morphology: Leaves erect, lanceolate, broadest in their basal part, acute at the apex, avg. Heslop-Harrison index: 1.1, spur usually short and thin.
Chromosome counts and ploidy level: 2n = 4x = 80.
Habitat and ecology: Sedge and peat-moss vegetation of the raised bogs and wet heath.
Distribution: Northern Lowlands. Germany, Netherlands.
Threat status: Unknown.
Dactylorhiza maculata subsp. fuchsii (Druce) Hyl.,
Nord. Kärlväxtfl. 2: 238 (1966)
≡ Orchis fuchsii Druce, Rep. Bot. Soc. Exch. Club Brit. Isles 4(1): 105 (1915)
≡ Dactylorhiza fuchsii (Druce) Soó, Nom. Nov. Gen. Dactylorhiza 8 (1962)
= Dactylorhiza maculata subsp. austriaca Vöth, Linzer Biol. Beitr. 10(1): 190 (1978)
Type: ‘[Great Britain] Challow Berks’, June 1895, G. C. Druce, OXF 6463 (digital image!; lectotype Vermeulen 1947: 147).
Morphology: Leaves obovate to oblanceolate, relatively broad, obtuse at the apex, lip purple to white, anther caps purple, avg. Heslop-Harison index: 1.4; populations consist of various proportions of purple-flowered plants with spotted leaves and white-flowered plants with unspotted leaves.
Chromosome counts and ploidy level: 2n = 2x = 40, 2n ~ 3x, 2n = 4x = 80.
Habitat and ecology: Broad-leaved and coniferous forests, soft-water springs, forest roadside ditches, wet to mesic mown meadows, moss-sedge vegetation.
Distribution: Throughout temperate Europe and Asia (Eccarius 2016), but regionally rare or absent (e.g. Pannonian Basin, Balkan Peninsula).
Threat status: Czechia: NT. Germany: ‘V-Vornwarnliste’ (Metzing et al. 2018). Hungary: VU (Király 2007). Poland: VU (Zarzycki and Szeląg 2006). Slovakia: NT (Eliáš et al. 2015).
Dactylorhiza maculata (L.) Soó subsp. maculata
= Orchis maculata subsp. ericetorum E. F. Linton, Fl. Bournemouth 208 (1900) ≡ Dactylorhiza ericetorum (Linton) Aver., Bot. Zhurn. 67(3): 309 (1982)
Type: Sweden, unknown locality in the surroundings of Uppsala, sine dato, C. Linnaeus, LINN 1054 (digital image!; lectotype Vermeulen 1947: 130).
Morphology: Leaves narrowly oblanceolate, widest in their middle part, acute or subacute at the apex, avg. Heslop-Harrison index : 1.3.
Chromosome counts and ploidy level: 2n = 4x = 80 (chromosome counts: e.g. Heslop-Harrison 1951; Jagiełło and Lankosz-Mróz, 1988; Aagaard et al. 2005).
Habitat and ecology: Sedge-moss vegetation of calcareous or acidic, usually mineral-rich fens.
Distribution: Atlantic and subatlantic Europe and Fennoscandia, less frequently in Central and East Europe to West Siberia (Eccarius 2016).
Threat status: Czechia: EN B1ab(ii,iii,iv)+2ab(ii,iii,iv). Hungary: VU (Király 2007). Poland: VU (Zarzycki and Szeląg 2006). Slovakia: EN (Eliáš et al. 2015). In Hungary and Poland, the evaluation relates to the species D. maculata, which may include some taxa here recognized as separate subspecies.
Dactylorhiza maculata subsp. sooana Borsos ex Batoušek, Taraška et Trávn.,
Plant Syst. Evol. 307: 51(16) (2021)
– Dactylorhiza fuchsii subsp. sooana Borsos, Acta Bot. Acad. Hung. 5: 324 (1959), nom. inval. (ICN Art. 40.1)
Type (holotype): ‘Slovakia, Štiavnické vrchy Hills, Banský Studenec Village, meadow in the valley of the Bystrý potok brook east of the village, 655 m.a. s. l., 48° 26′ 31″ N, 19° 00′ 49" E’, 13 June 2017, leg. excursion group, OL 37871!
Morphology: Leaves obovate to oblanceolate, relatively broad, obtuse at the apex, always spotted, lip white with or without markings, anther caps white, avg. Heslop-Harrison index: 1.3.
Chromosome counts and ploidy level: 2n = 2x = 40.
Habitat and ecology: Wet to meso-xeric mown meadows, secondary mat-grass swards, basiphilous beech forests and oak forests in warm cool-temperate regions.
Distribution: Endemic to the West Carpathians. Czechia, Hungary, Slovakia. Reports from other parts of the Carpathians (e.g. Loya 2015) must be examined.
Threat status: Czechia: EN B1ab(ii,iii,iv)+2ab(ii,iii,iv); C2a(i). Slovakia: NT (Eliáš et al. 2015). Hungary: VU; evaluated within D. fuchsii (Király 2007).
Dactylorhiza maculata subsp. sudetica (Poech ex Rchb.f.) Vöth,
Linzer. Biol. Beitr. 12(2): 430 (1980)
≡ Orchis maculata var. sudetica Poech ex Rchb.f., Icon. Fl. Germ. Helv. 13/14: 66, tab. 56 (1850)
≡ Dactylorhiza fuchsii subsp. sudetica (Poech ex Rchb.f.) Verm., Orchideeën 37(3): 78 (1975)
≡ Dactylorhiza sudetica (Poech ex Rchb.f.) Aver., Bot. Zhurn. 67(3): 310 (1982)
– Dactylorhiza fuchsii subsp. psychrophila auct. non (Schltr.) Holub 1964: Procházka, Zpr. Čes. Bot. Společ. 14: 11 (1979)
– Dactylorhiza fuchsii var. psychrophila auct. non (Schltr.) Soó 1962: Kubát, Flora of the Czech Republic 8: 520 (2010)
Type: Rchb. f., Icon. Fl. Germ. Helv. 13/14: tab. 56. 1850 (lectotype Baumann et al. 2002: 144).
Epitype (designated here): sine loco [Sudeten Mts], sine dato, leg. J. A. Poech, W 0028325!
Note: The protologue contains both an illustration and a reference to the herbarium specimen. The first was selected as a lectotype by Baumann et al. (2002). This typification was later questioned by Eccarius (2011), but it conforms to the ICN (Turland et al. 2018). The illustration must be thus regarded as lectotype, while the herbarium specimen is here designated as an epitype.
Morphology: Dwarf plants with the stem height never exceeding 40 cm, usually with 2–3 elliptic, oblanceolate to obovate sheathing leaves with subacute to obtuse apices, avg. Heslop-Harrison index: 1.2, flowers often darkly reddish-purple, frequent anthocyanin pigmentation of bracts, ovaries and inflorescence axis.
Chromosome counts and ploidy level: 2n = 4x = 80 (chromosome counts: Krahulcová 2003)
Habitat and ecology: Subalpine oligotrophic water-springs.
Distribution: Endemic to the Krkonoše Mts. Czechia, Poland.
Threat status: Czechia: EN B1ab(iii)+2ab(iii). Poland: not evaluated (cf. Zarzycki and Szeląg 2006).
Note: Unlike other taxa of the D. maculata agg. classified within the category of EN in Czechia, D. maculata subsp. sudetica probably did not undergo a significant decrease of its population size, and it also does not exhibit extreme fluctuations (i.e. greater than one order of magnitude; IUCN 2012a) in the number of individuals, as it was assumed in the national Red List (Grulich 2017). Yet, it occurs in the subalpine belt where it faces both climate change and over-tourism (Flousek 2019; Erlebach and Romportl 2021), prospectively leading to changes in habitat extent and quality.
Dactylorhiza maculata subsp. transsilvanica (Schur) Soó,
Nom. Nov. Gen. Dactylorhiza 7 (1962)
≡ Orchis transsilvanica Schur, Verh. Mitth. Siebenbürg. Vereins Naturwiss. Hermannstadt 4: 72 (1853)
≡ Dactylorhiza transsilvanica (Schur) Aver., Bot. Zhurn. 67(3): 309 (1982)
≡ Dactylorhiza maculata var. transsilvanica (Schur) P. Delforge, Naturalistes Belges 81(4): 397 (2000)
Type: ‘Auf Moorboden am Schewechbach’, 9 June 1853, leg. P. J. F. Schur, LW (digital image!; lectotype Klein and Deutsch 2005: 231).
Morphology: Leaves oblanceolate to narrowly oblanceolate, usually subacute or obtuse at the apex, avg. Heslop-Harrison index: 1.2; populations formed by a significant proportion of white-flowered plants with unspotted leaves, but often including also purple-flowered plants with spotted leaves, as well as continuous transitions between these two forms.
Chromosome counts and ploidy level: 2n = 4x = 80 (chromosome counts: Klein and Deutsch 2005; Petrova et al. 2009); rarely 2n ~ 6x.
Habitat and ecology: Sedge-moss fens, wet to mesic mown meadows and pastures, secondary mat-grass swards and meso-xerophytic grasslands, usually calcareous, mineral-rich and nutrient-poor soils.
Distribution: Bulgaria, Czechia, Romania, Slovakia, Slovenia; mentioned from Hungary (Molnár and Csábi 2021), herbarium specimens of uncertain identity collected in Bosnia and Herzegovina (Loschnigg 1929, OLM !) and Montenegro (Rohlena 1903, PRC !). Carpathians, Dinarides, Stara Planina Mts and Pannonian Basin.
Threat status: Czechia: EN B1ab(ii,iii,iv)+2ab(ii,iii,iv); C2a(i). Slovakia: CR (Eliáš et al. 2015). Hungary: EX; evaluated within D. maculata (Király 2007).
Determination key to subspecies of D. maculata in Central Europe
The key provided here serves to determine populations of D. maculata in Central Europe. It gives the most frequent, average and extreme (10–90 percentile, minimum and/or maximum in brackets) values of particular traits, not necessarily individual attributes of each plant. It should therefore not be applied to single plants because of extensive individual variability within the group. Instead, each population must be considered as a whole, and single plants with aberrant phenotypes should be regarded as part of its variation. Populations which do not merit criteria to be assigned to any subspecies should be referred to as D. maculata s. lat. or, possibly, as transitional populations among specific subspecies.
(1a) Lowermost well-developed leaf oblong, oblanceolate to obovate, max. 5.2(–7.5)× longer than wide, usually with obtuse apex; avg. Heslop-Harrison index ≥ 1.3; 2n = 2x, 3x, 4x ............................ 2
(1b) Lowermost well-developed leaf linear, oblanceolate to lanceolate, up to 9.8(–21.7)× longer than wide, with acute, subacute or obtuse apex; avg. Heslop-Harrison index ≤ 1.3; 2n = 4x (rarely 6x) ...... 3
(2a) Leaves always spotted (intensity of leaves spotting does not correlate with intensity of flower colouration and tepal markings); tepals white or, rarely, pink, lip and anther caps nearly always white (regardless intensity of lip markings); 2n = 2x. – Mesic meadows, broad-leaved woodlands and forests; Carpathians ....................................................................................................... subsp. sooana
(2b) Leaves spotted or unspotted (intensity of leaves spotting positively correlates with intensity of flower colouration and markings); tepals and lip pink or, less often, white, anther caps always purple (excl. achromatic individuals); 2n = 2x, 3x, 4x. – Forests, meadows, roadside ditches; widespread ...... ………………………………………………………………………………………………….. subsp. fuchsii
(3a) Lowermost well-developed leaf (2.1–)3.3–6.9(–12.5)× longer than wide, predominantly obtuse or subacute at the apex ........................................................................................................... 4
(3b) Lowermost well-developed leaf (3.0–)4.7–12.5(–21.7)× longer than wide, predominantly acute to subacute at the apex .......................................................................................................................... 5
(4a) Plants up to 36(–40) cm high, most often with 5 cauline (incl. bract-like) leaves; lowermost well-developed leaf up to 10(–13) cm long, usually spotted; inflorescence axes, bracts and ovaries usually with purple anthocyanin pigmentation; lip pink to darkly (reddish-)purple with markings (flower colouration often resembling that of D. majalis), only rarely white without markings (achromatic plants). – Subalpine springs and grasslands; endemic to the Krkonoše Mts …………………………………………………………………………………………………………………………... subsp. sudetica
(4b) Plants up to 56(–67) cm high, most often with 7 cauline (incl. bract-like) leaves; lowermost well-developed leaf up to 14(–20) cm long, spotted or unspotted; inflorescence axes, bracts and ovaries usually green without anthocyanin pigmentation; lip white or pink, with or without markings. – Populations consisting predominantly, or at least partly of white-flowered plants with unspotted leaves. Mesic to wet meadows and fens; Carpathians, Dinarides, Stara Planina Mts, Pannonia ……………………………………………………………………............................................. subsp. transsilvanica
(5a) Leaves lanceolate, erect, usually widest in their basal half; Heslop-Harrison index ≤ 1.1(–1.2), spur thin and short, 0.5–0.8(–0.9)× as long as the lip. Leaves with pale spots or unspotted, rarely with bold spots. – Wet heaths; subatlantic West and Central Europe ……....................................... subsp. elodes
(5b) Leaves linear to oblanceolate, erect or spread out, usually widest in their upper half; Heslop-Harrison index ≤ 1.4(–2.1), spur relatively thick and long, (0.6–)0.9–1.3(–1.7)× as long as the lip. – Leaves with pale to bold spots or unspotted ………………………………………………………….............. 6
(6a) 2nd well-developed leaf from the base of the stem up to 21(–28) cm long, (6–)8–19(–23)× longer than wide, narrowly linear with ± parallel margins in the widest part of the leaf, nearly always acute at the apex. – Open pine and spruce woods on mires, rarely open oligotrophic mires ...................................................................... subsp. averyanovii
(6b) 2nd well-developed leaf from the base of the stem up to 17(–22) cm long, (1–)5–11(–14)× longer than wide, oblanceolate with convex margins in the widest part of the leaf, acute to subacute, rarely obtuse at the apex. – Usually non-woodland habitats ..................................................................... 7
(7a) Stem less densely foliated (avg. 1.7 leaves per 10 cm of the stem length); leaves rather spread out, oblanceolate or lanceolate with the widest place around their middle part; lowermost well-developed leaf typically acute or subacute, rarely obtuse at the apex; inflorescence sparse to dense (compact), lip white to pink, rarely purple, with or without markings, spur usually not conspicuously robust, ca 8.7 mm long and 2.1 mm wide, pink to purple, less often white. – Fens, sedge-moss vegetation; rare but widespread. ………………………………………………………………………………........................... subsp. maculata
(7b) Stem more densely foliated (avg. 2.4 leaves per 10 cm of stem length); leaves rather erect, narrowly oblanceolate with the widest place in their upper half; lowermost well-developed leaf typically subacute, rarely obtuse or acute at the apex; inflorescence usually sparse (not compact), lip purple to darkly (reddish-)purple, with bold or, rarely, pale markings, spur conspicuously robust, ca 9.3 mm long and 2.4 mm wide, purple (flower colouration and spur shape somewhat resembling that of D. traunsteineri). – Endemic to the Oravské Beskydy and Tatry Mts ……………....... subsp. arcana
Change history
23 March 2024
A Correction to this paper has been published: https://doi.org/10.1007/s12224-024-09447-8
References
Aagaard SMD, Såstad SM, Greilhuber J, Moen A (2005) A secondary hybrid zone between diploid Dactylorhiza incarnata ssp. cruenta and allotetraploid D. lapponica (Orchidaceae). Heredity 94:488–496
Addinsoft (2022) XLSTAT statistical and data analysis solution. Long Island, NY, USA
Averyanov LV (1983) The genus Dactylorhiza (Orchidaceae) in the USSR. Bot Zhurn 68:1160–1167 [in Russian]
Averyanov LV (1990) A review of the genus Dactylorhiza. Timber Press, Portland
Balao F, Tannhäuser M, Lorenzo MT, Hedrén M, Paun O (2016) Genetic differentiation and admixture between sibling allopolyploids in the Dactylorhiza majalis complex. Heredity 116:351–361
Barker M, Rayens W (2003) Partial least squares for discrimination. J Chemometr 17:166–173
Bateman RM (2021) Challenges of applying monophyly in the phylogenetic shallows: taxonomic reappraisal of the Dactylorhiza maculata group. Kew Bull 76:675–704
Bateman RM, Denholm I (1983) A reappraisal of the British and Irish dactylorchids, 1. The tetraploid marsh-orchids. Watsonia 14:347–376
Bateman RM, Denholm I (1988) A reappraisal of the British and Irish dactylorchids, 3. The spotted-orchids. Watsonia 17:319–349
Bateman RM, Denholm I (2003) The Heath Spotted-orchid (Dactylorhiza maculata (L.) Soó) in the British Isles: a cautionary case-study in delimitating infraspecific taxa and inferring their evolutionary relationships. J Eur Orch 35:3–36
Bateman RM, Hollingsworth PM, Preston J, Yi-Bo L, Pridgeon AM, Chase MW (2003) Molecular phylogenetics and evolution of Orchidinae and selected Habenariinae (Orchidaceae). Bot J Linn Soc 142:1–40
Bateman RM, Murphy ARM, Hollingsworth PM, Hart ML, Denholm I, Rudall PJ (2018) Molecular and morphological phylogenetics of the digitate-tubered clade within subtribe Orchidinae s.s. (Orchidaceae: Orchideae). Kew Bull 73:54
Baumann H, Künkele S, Lorenz R (2002) Taxonomische Liste der Orchideen Deutschlands. J Eur Orch 34:129–206
Baumann H, Künkele S, Lorenz R (2004) Taxonomische Liste der Orchideen Deutschlands – Nachtrag. J Eur Orch 36:769–780
Bernátová D, Škovirová K, Kliment J, Obuch J, Topercer J (1993) Dactylorhiza maculata (L.) Soó subsp. transsilvanica (Schur) Soó in the Kremnické vrchy Mts. Biológia 48:389–390
Borsos O (1959) Dactylorchis fuchsii Druce et son affinité dans les flores Hongroise et Carpatique. Acta Bot Acad Sci Hung 5:321–325
Borsos O (1961) Geobotanische Monographie der Orchideen der pannonischen und karpatischen Flora V. Ann Univ Sci Budapest Rolando Eotvos, Sect Biol 4:51–82
Brandrud MK, Baar J, Lorenzo MT, Athanasiadis A, Bateman RM, Chase MW, Hedrén M, Paun O (2020) Phylogenomic relationships of diploids and the origins of allotetraploids in Dactylorhiza (Orchidaceae). Syst Biol 69:91–109
Bureš L (2013) Chráněné a ohrožené rostliny Chráněné krajinné oblasti Jeseníky [Protected and rare plants of the PLA Jeseníky]. Rubico, Olomouc [in Czech]
Conti F, Abbate G, Alessandrini A, Blasi C (2005) An annotated checklist of the Italian vascular flora. Palombi e Partner, Roma
Delforge P (2006) Orchids of Europe, North Africa and the Middle East. A&C Black, London
Devillers P, Devillers-Terschuren J (2000) Dactylorhiza sudetica (Pöch ex Rchb. fil. 1851) Averyanov 1982 dans les monts des Géants. Naturalistes Belges 81:331–338
Devos N, Tyteca D, Raspé O, Wesselingh RA, Jacquemart A-L (2003) Patterns of chloroplast diversity among western European Dactylorhiza species (Orchidaceae). Pl Sys Evol 243:85–97
Devos N, Oh S-H, Raspé O, Jacquemart A-L, Manos PS (2005) Nuclear ribosomal DNA sequence variation and evolution of spotted marsh-orchids (Dactylorhiza maculata group). Molec Phylogenet Evol 36: 568–580
Doležel J, Greilhuber J, Lucretti S, Meister A, Lysák MA, Nardi L, Obermayer R (1998) Plant genome size estimation by flow cytometry: inter-laboratory comparison. Ann Bot (Oxford) 82 (Suppl. A):17–26
Doležel J, Greilhuber J, Suda J (2007) Estimation of nuclear DNA content in plants using flow cytometry. Nature Protocols 2:2233–2244
Druce GC (1915) Orchis maculata L. and O. fuchsii. Rep Bot Exch Club Soc Brit Isles 4:99–108
Dufrêne M, Gathoye J-L, Tyteca D (1991) Biostatistical studies on western European Dactylorhiza (Orchidaceae) – the D. maculata group. Pl Syst Evol 175:55–72
Dusak F, Prat D (2010) Atlas des Orchidées de France. Muséum national d'Histoire naturelle, Paris
Eccarius W (2011) Zur Typisierung einiger Orchideen Taxa. Ber Arbeitskreis Heimische Orchid 28:86–103
Eccarius W (2016) Die Orchideengattung Dactylorhiza. Phylogenie, Taxonomie, Morphologie, Biologie, Verbreitung, Ökologie und Hybridisation. W. Eccarius, Eisenach
Eliáš P jun, Dítě D, Kliment J, Hrivnák R, Feráková V (2015) Red list of ferns and flowering plants of Slovakia, 5th edition (October 2014). Biologia 70:218–228
Erlebach M, Romportl D (2021) Časoprostorová distribuce turismu v Krkonoších a jeho environmentální dopady [Spatio-temporal distribution of tourism in the Krkonoše Mts and its environmental impacts]. Opera Corcontica 58:5–25 [in Czech]
Fick SE, Hijmans RJ (2017) WorldClim 2: new 1-km spatial resolution climate surfaces for global land areas. Int J Climatol 37:4302–4315
Fischer MA, Oswald K, Adler W (2008) Exkursionsflora für Österreich, Liechtenstein und Südtirol, Ed. 3. Biologiezentrum der Oberösterreichischen Landesmuseen, Linz
Flousek J (2019) Krkonoše a klimatická změna [The Giant Mts and the climate change]. Fórum ochrany přírody 4/2019:12–15 [in Czech]
Gajdošová Z, Svitok M, Cetlová V, Mártonfiová L, Kučera J, Kolarčik V, Hurdu BI, Sîrbu IM, Turisová I, Turis P, Slovák M (2023) Incidence and evolutionary relevance of autotriploid cytotypes in a relict member of the genus Daphne (Thymelaeaceae). AoB Plants 15:056
Galbraith DW, Harkins KR, Maddox JM, Ayres NM, Sharma DP, Firoozabady E (1983) Rapid flow cytometric analysis of the cell cycle in intact plant tissues. Science 220:1049–1051
Gigord LDB, Macnair MR, Smithson A (2001) Negative frequency-dependent selection maintains a dramatic flower color polymorphism in the rewardless orchid Dactylorhiza sambucina (L.) Soó. Proc Natl Acad Sci USA 98:6253–6255
Grisebach A (1845) Über die Bildung des Torfes in den Emsmooren aus deren unveränderter Pflanzendecke. Nebst Bemerkungen über die Culturfähigkeit des Bourtanger Hochmoors. In Anonymus, Göttinger Studien. Vandenhoeck und Ruprecht, Göttingen, pp 255-370
Grulich V (2017) The Red List of vascular plants of the Czech Republic. Příroda 35:75–132 [in Czech]
Hansson S (1985) Orkidéer i svensk natur. AB Wiken, Höganäs [in Swedish]
Hanušová K, Čertner M, Urfus T, Koutecký P, Košnar J, Rothfels CJ, Jarolímová V, Ptáček J, Ekrt L (2019) Widespread co-occurrence of multiple ploidy levels in fragile ferns (Cystopteris fragilis complex; Cystopteridaceae) likely stems from similar ecology of cytotypes, their efficient dispersal and inter-ploidy hybridization. Ann Bot (Oxford) 123:845–855
Hassler M, Muer T (2022) Flora Germanica. Alle Farn- und Blütenpflanzen Deutschlands in Text und Bild. Band 1, Einführung, Lebensräume und Statistik. Artenteil 1, Bärlappe und Verwandte, Farne, Basale Angiospermen, Einkeimblättrige, Zweikeimblättrige. Verlag Regionalkultur, Ubstadt-Weiher
Hedrén M (1996) Genetic differentiation, polyploidization and hybridization in northern European Dactylorhiza (Orchidaceae): evidence from allozyme markers. Pla Syst Evol 201:31–55
Hedrén M (2001) Systematics of the Dactylorhiza euxina/incarnata/maculata polyploid complex (Orchidaceae) in Turkey: evidence from allozyme data. Pl Syst Evol 229:23–44
Hedrén M, Fay MF, Chase MW (2001) Amplified fragment length polymorphisms (AFLP) reveal details of polyploid evolution in Dactylorhiza (Orchidaceae). Amer J Bot 88:1868–1880
Hedrén M, Nordström S, Ståhlberg D (2008) Polyploid evolution and plastid DNA variation in the Dactylorhiza incarnata/maculata complex (Orchidaceae) in Scandinavia. Molec Ecol 17:5075–5091
Hedrén M (2003) Plastid DNA variation in the Dactylorhiza incarnata/maculata polyploid complex and the origin of allotetraploid D. sphagnicola (Orchidaceae). Molec Ecol 12:2669–2680
Hengl T, de Jesus JM, Heuvelink GBM, Gonzalez MR, Kilibarda M, Blagotić A, Shangguan W, Wright MN, Geng X, Bauer-Marschalliner B, Guevara MA, Vargas R, MacMillan RA, Batjes NH, Leenaars JGB, Ribeiro E, Wheeler I, Mantel S, Kempen B (2017) SoilGrids250m: global gridded soil information based on machine learning. PLOS One12:e0169748
Heslop-Harrison J (1948) Field studies in Orchis L. I. The structure of Dactylorchid populations on certain islands in the Inner and Outer Hebrides. Trans Roy Soc Edinburgh 35:26–66
Heslop-Harrison J (1951) A comparison of some Swedish and British forms of Orchis maculata L. sens. lat. Svensk Bot Tidskr 45:608–635
Heslop-Harrison J (1968) Genetic system and ecological habit as factors in Dactylorchid variation. Jahresber Naturwiss Vereins Wuppertal 21:20–27
Hülber K, Sonnleitner M, Suda J, Krejčíková J, Schönswetter P, Schneeweiss GM, Winkler M (2015) Ecological differentiation, lack of hybrids involving diploids, and asymmetric gene flow between polyploids in narrow contact zones of Senecio carniolicus (syn. Jacobaea carniolica, Asteraceae). Ecol Evol 5:1224–1234
IUCN (2012a) IUCN Red List categories and criteria: Version 3.1. 2nd ed. Gland, Switzerland and Cambridge, UK
IUCN (2012b) Guidelines for application of IUCN Red List criteria at regional and national levels: Version 4.0. Gland, Switzerland and Cambridge, UK
Jäger EJ, Werner K (2006) Rothmaler – Exkursionsflora von Deutschland. 4. Gefässpflanzen: Kritischer Band. Spektrum Akademischer Verlag, München
Jagiełło M (1988) Analysis of population variability and distribution of species from the Dactylorhiza maculata group (Orchidaceae) in Poland. Fragm Florist Geobot 31–32:333–383
Jagiełło M (1990) Variability and distribution of some species from the genus Dactylorhiza Necker ex Nevski in Poland. Acta Univ Wratislav, Prace bot 1055:45–55
Jagiełło M, Lankosz-Mróz M (1988) Cytotaxonomic studies in the Dactylorhiza maculata (L.) Soó group in Poland (Orchidaceae). Fragm Florist Geobot 31–32:385–394
Jersáková J, Kindlmann P, Renner SS (2006) Is the colour dimorphism in Dactylorhiza sambucina maintained by differential seed viability instead of frequency-dependent selection? Folia Geobot 41:61–76
Joffard N, Buatois B, Arnal V, Véla E, Montgelard C, Schatz B (2022) Delimiting species in the taxonomically challenging orchid section Pseudophrys: Bayesian analyses of genetic and phenotypic data. Frontiers Ecol Evol 10:1058550
Kadereit JW (2015) The geography of hybrid speciation in plants. Taxon 64:673–687
Kaplan Z, Danihelka J, Chrtek J Jr, Kirschner J, Kubát K, Štech M, Štěpánek J [eds] (2019) Klíč ke květeně České republiky [Key to the flora of the Czech Republic]. Ed. 2. Academia, Praha [in Czech]
Kaplan Z, Danihelka J, Šumberová K, Chrtek J Jr, Rotreklová O, Ekrt L, Štěpánková J, Taraška V, Trávníček B, Prančl J, Ducháček M, Hroneš M, Kobrlová L, Horák D, Wild J (2017) Distributions of vascular plants in the Czech Republic. Part 5. Preslia 89:333–439
Keller G, Schlechter R (1928) Monographie und Iconographie der Orchideen Europas und des Mittelmeergebietes. Verlag des Repertoriums, Berlin-Dahlem
Király G (2007) Vörös Lista. A magyarországi edényes flóra veszélyeztetett fajai [Red list of the vascular flora of Hungary]. Saját kiadás, Sopron [in Hungarian]
Kirillova IA, Dubrovskiy YA, Degteva SV, Novakovskiy AB (2022) Ecological and habitat ranges of orchids in the northernmost regions of their distribution areas: a case study from Ural Mountains, Russia. Pl Diversity 45:211–218
Klášterský I, Hrabětová-Uhrová A, Duda J (1982) Dějiny floristického výzkumu v Čechách, na Moravě a ve Slezsku [History of the floristic research in Bohemia, Moravia and Silesia]. Vol. 1. Severočeskou přírodou, suppl. 1982/1:133–242 [in Czech]
Klein E, Deutsch G (2005) Dactylorhiza transsilvanica (Schur) Averyanov ist definitiv keine weitere diploide Sippe aus dem Dactylorhiza maculata Komplex. J Eur Och 37:229–233
Knotek A, Konečná V, Wos G, Požárová D, Šrámková G, Bohutínská M, Zeisek V, Marhold K, Kolář F. (2020) Parallel alpine differentiation in Arabidopsis arenosa. Frontiers Pl Sci 11:561526
Kobrlová L, Duchoslav M, Hroneš M (2022) Morphological, ecological and geographic differences between diploids and tetraploids of Symphytum officinale (Boraginaceae) justify both cytotypes as separate species. AoB Plants 14:plac028
Kolář F, Čertner M, Suda J, Schönswetter P, Husband BC (2017) Mixed-ploidy species: progress and opportunities in polyploid research. Trends Pl Sci 22:1041–1055
Koutecký P (2015) MorphoTools: a set of R functions for morphometric analysis. Pl Syst Evol 301:1115–1121
Koutecký P, Prančl J, Košnar J, Koutecká E, Hanzlíčková J, Lučanová M, Nejedlá M, Kaplan Z (2022) Waking up from a taxonomist’s nightmare: emerging structure of Ranunculus section Batrachium (Ranunculaceae) in central Europe based on molecular data and genome sizes. Bot J Linn Soc 198:417–437
Kreutz CAJ (2004) Kompendium der Europäischen Orchideen. Kreutz Publishers, Landgraaf
Krahulcová A (2003) Chromosome numbers in selected monocotyledons (Czech Republic, Hungary and Slovakia). Preslia 75:97–113
Kubát K (2010) Dactylorhiza Nevski – prstnatec [Dactylorhiza Nevski – marsh and spotted orchid]. In Štěpánková J, Chrtek J, Kaplan Z (eds) Květena České republiky 8. Academia, Praha, pp 502–523 [in Czech]
Legendre P, Legendre L (1998) Numerical ecology. 2nd English Edition. Elsevier, Amsterdam
Linné C (1753) Species plantarum. Tom. 2. Laurentius Salvius, Stockholm
Linton EF (1900) Flora of Bournemouth, including the Isle of Purbeck. Turnbull and Spears, Bournemouth
Lord RM, Richards AJ (1977) A hybrid swarm between the diploid Dactylorhiza fuchsii (Druce) Soó and the tetraploid D. purpurella (T. & T. A. Steph.) Soó in Durham. Watsonia 11:205–210
Lowry DB (2012) Ecotypes and the controversy over stages in the formation of new species. Biol J Linn Soc 106:241–257
Loya VV (2015) Dactylorhiza fuchsii (Druce) Soó subsp. sooana (Borsos) Borsos u flori Zakarpattya [Dactylorhiza fuchsii (Druce) Soó subsp. sooana (Borsos) Borsos in the flora of the Zakarpattya region]. Naukoviy visnik Uzhgorodskogo universitetu, seria Biologia 38–39:36–38 [in Ukrainian]
Lysák MA, Doležel J (1998) Estimation of nuclear DNA content in Sesleria (Poaceae). Caryologia 52:123–132
Majeský Ľ, Hroneš M, Kitner M, Válová L, Mártonfiová L, Płachno BJ, Conti F, Dančák M (2022) Pinguicula vulgaris in central Europe: When does one species turn into another? Preslia 94:275–304
Mendez-Castro FE, Conti L, Chytrý M, Jiménez-Alfaro B, Hájek M, Horsák M, Zelený D, Malavasi M, Ottaviani G (2021) What defines insularity for plants in edaphic islands? Ecography 44:1249–1258
Metzing D, Garve E, Matzke-Hajek G, Adler J, Bleeker W, Breunig T, Caspari S, Dunkel FG, Fritsch R, Gottschlich G, Gregor T, Hand R, Hauck M, Korsch H, Meierott L, Meyer N, Renker C, Romahn K, Schulz D, Täuber T, Uhlemann I, Welk E, van de Weyer K, Wörz A, Zahlheimer W, Zehm A, Zimmermann F (2018) Rote Liste und Gesamtartenliste der Farn- und Blütenpflanzen (Trachaeophyta) Deutschlands. Naturschutz und Biologische Vielfalt 70:13–358
Mirek Z, Piękoś-Mirkowa H, Zając A, Zając M (2020) Vascular plants of Poland. An annotated checklist. W. Szafer Institute of Botany, Polish Academy of Sciences, Kraków
Molnár VA (2011) Magyarország orchideáinak atlasza [Atlas of Orchids of Hungary]. Kossuth Kiadó, Budapest [in Hungarian]
Molnár VA, Csábi M (2021) Magyarország orchideái [Orchids of Hungary]. Debreceni Egyetem TTK Növénytani Tanszék, Debrecen [in Hungarian]
Mucina L, Bültmann H, Dierßen K, Theurillat JP, Raus, T, Čarni A, Šumberová K, Willner W, Dengler J, García RG, Chytrý M, Hájek M, Di Pietro R, Iakushenko D, Pallas J, Daniëls FJA, Bergmeier E, Santos Guerra A, Ermakov N, Valachovič M, Schaminée JHJ, Lysenko T, Didukh YP, Pignatti S, Rodwell JS, Capelo J, Weber HE, Solomeshch A, Dimopoulos P, Aguiar C, Hennekens SM, Tichý L (2016) Vegetation of Europe: hierarchical floristic classification system of vascular plant, bryophyte, lichen, and algal communities. Appl Veg Sci 19:3–264
Müller F, Ritz CM, Welk E, Wesche K (2021) Rothmaler – Exkursionsflora von Deutschland. Gefässpflanzen: Grundband. Ed. 22. Springer Spektrum Berlin, Heidelberg
Naczk AM, Górniak M, Szlachetko DL, Ziętara MS (2015) Plastid DNA haplotype diversity and morphological variation in the Dactylorhiza incarnata/maculata complex (Orchidaceae) in northern Poland. Bot J Linn Soc 178:121–137
Naimi B, Hamm Na, Groen TA, Skidmore AK, Toxopeus AG (2014) Where is positional uncertainty a problem for species distribution modelling. Ecography 37:191–203
NCSS (2013) NCSS 9 Statistical Software. NCSS, LLC. Kaysville, Utah, USA
Nordström S, Hedrén M (2008) Genetic differentiation and postglacial migration of the Dactylorhiza majalis ssp. traunsteineri/lapponica complex into Fennoscandia. Pl Syst Evol 276:73–87
Nordström S, Hedrén M (2009) Evolution, phylogeography and taxonomy of allopolyploid Dactylorhiza (Orchidaceae) and its implications for conservation. Nordic J Bot 27:548–556
Paun O, Bateman RM, Fay MF, Hedrén M, Civeyrel L, Chase MW (2011) Stable epigenetic effects impact adaptation in allopolyploid orchids (Dactylorhiza: Orchidaceae). Molec Biol Evol 27:2465–2473
Pedersen HÆ (1998) Species concept and guidelines for infraspecific taxonomic ranking in Dactylorhiza (Orchidaceae). Nordic J Bot 18:289–310
Pedersen HÆ (2006) Systematics and evolution of the Dactylorhiza romana/sambucina polyploid complex (Orchidaceae). Bot J Linn Soc 152:405–434
Pedersen HÆ, Hedrén M, Bateman RM (2003) Proposal to conserve the name Orchis majalis against O. elata, O. vestita and O. sesquipedalis (Dactylorhiza: Orchidinae: Orchidaceae). Taxon 52:633–634
Petrova AS, Vladimirov V, Stoyanov Y (2009) Dactylorhiza maculata subsp. transsilvanica (Orchidaceae): new for the Bulgarian flora. Phytol Balcan 15:389–392
Pillon Y, Fay MF, Hedrén M, Bateman RM, Devey DS, Shipunov AB, van der Bank M, Chase MW (2007) Evolution and temporal diversification of western European polyploid species complexes in Dactylorhiza (Orchidaceae). Taxon 56:1185–1208
Pillon Y, Fay MF, Shipunov AB, Chase MW (2006) Species diversity versus phylogenetic diversity: a practical study in the taxonomically difficult genus Dactylorhiza (Orchidaceae). Biol Conservation 129:4–13
Ponert J (2019) Dactylorhiza Nevski – prstnatec (vstavač). In Kaplan Z, Danihelka J, Chrtek J Jr, Kirschner J, Kubát K, Štech M, Štěpánek J (eds) Klíč ke květeně České republiky [Key to the Flora of the Czech Republic]. 2nd ed. Academia, Praha, pp 183–187 [in Czech]
Popelka O, Sochor M, Duchoslav M (2019) Reciprocal hybridization between diploid Ficaria calthifolia and tetraploid Ficaria verna subsp. verna: evidence from experimental crossing, genome size and molecular markers. Bot J Linn Soc 189:293–310
Procházka F (1979) Okruh prstnatce plamatého (Dactylorhiza maculata agg.) v Československu [Dactylorhiza maculata group in Czechoslovakia]. Zprávy Českoslov Bot Společn 14:9–12 [in Czech]
Průša D (2019) Orchideje České republiky [Orchids of the Czech Republic]. 2nd ed. CPress, Brno [in Czech]
R Core Team (2022). R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria
Redl K (2003) Wildwachsende Orchideen in Österreich – faszinierend und schützenswert, 3rd edn. K. Redl Eigenverlag, Altenmarkt
Reichenbach HG (1850) Icones florae Germanicae et Helveticae, simul Pedemontanae, Tirolensis, Istriacae, Dalmaticae, Austriacae, Hungaricae, Transylvanicae, Moravicae, Borussicae, Holsaticae, Belgicae, Hollandicae, ergo Mediae Europae. Vol. 13–14. F. Hofmeister, Leipzig
Rohart F, Gautier B, Singh A, Lê Cao KA (2017) mixOmics: An R package for ‘omics feature selection and multiple data integration. PLOS Computat Biol 13:e1005752
Roy K, Valentine JW, Jablonski D, Kidwell, SM (1996) Scales of climatic variability and time averaging in Pleistocene biotas: implications for ecology and evolution. Trends Ecol Evol 11:458–463
Šafářová L, Duchoslav M (2010) Cytotype distribution in mixed populations of polyploid Allium oleraceum measured at a microgeographic scale. Preslia 82:107–126
Schur F (1853) Sertum florae Transsilvaniae sive Enumeratio systematica omnium plantarum, quae in Transsilvania sponte crescant et in usum hominum copiosus coluntur. Verh Mitth Siebenbürg Vereins Naturwiss Hermannstadt 4 (Suppl. 1):1–94
Scott ER, Crone EE (2021) Using the right tool for the job: the difference between unsupervised and supervised analyses of multivariate ecological data. Oecologia 196:13–25
Sczepanski S (2006) Zur Kenntnis einer bislang wenig beachteten Unterart von Dactylorhiza maculata (L.) Soó in Nordrhein-Westfalen: Dactylorhiza maculata subsp. elodes (Griseb.) Soó. J Eur Orch 38:867–896
Slatyer RA, Hirst M, Sexton JP (2013) Niche breadth predicts geographical range size: a general ecological pattern. Ecol Letters 16:1104–1114
Soó R (1980) Dactylorhiza Necker ex Nevski. In Tutin TG, Heywood VH, Burges NA, Moore DM, Valentine DH, Walters SM, Webb DA (eds) Flora Europaea, vol 5. Cambridge University Press, Cambridge, pp 335–337
Španiel S, Šlenker M, Melichárková A, Caboňová M, Šandalová M, Zeisek V, Marhold K, Zozomová-Lihová J (2023) Phylogenetic challenges in a recently diversified and polyploid-rich Alyssum (Brassicaceae) lineage: low divergence, reticulation and parallel polyploid speciation. Evolution 20:1–19
Ståhlberg D (2009) Habitat differentiation, hybridization and gene flow patterns in mixed populations of diploid and autotetraploid Dactylorhiza maculata s. l. (Orchidaceae). Evol Ecol 23:295–328
Ståhlberg D, Hedrén M (2008) Systematics and phylogeography of the Dactylorhiza maculata complex (Orchidaceae) in Scandinavia: insights from cytological, morphological and molecular data. Pl Syst Evol 273:107–132
Ståhlberg D, Hedrén M (2010) Evolutionary history of the Dactylorhiza maculata polyploid complex (Orchidaceae). Biol J Linn Soc 101:503–525
Ströhle W (2003) Nomenklatorische Liste der europäischen Orchideentaxa – Arten und Unterarten. J Eur Orch 35:771–860
Taraška V (2014) Karyological and morphological variability of the Dactylorhiza maculata group in the Czech Republic and western Slovakia. Ms. thesis. Palacký University, Olomouc
Taraška V, Batoušek P, Duchoslav M, Temsch EM, Weiss-Schneeweiss H, Trávníček B (2021) Morphological variability, cytotype diversity, and cytogeography of populations traditionally called Dactylorhiza fuchsii in Central Europe. Pl Syst Evol 307:51
ter Braak CJF, Šmilauer P (2012) CANOCO reference manual and User’s guide: software for ordination (version 5.0). Biometris, Wageningen
Trávníček P, Jersáková J, Kubátová B, Krejčíková J, Bateman RM, Lučanová M, Krajníková E, Těšitelová T, Štípková Z, Amardeilh JP, Brzosko E, Jermakowicz E, Cabanne O, Durka W, Efimov P, Hedrén M, Hermosilla C E, Kreutz K, Kull T, Marchand O, Rey M, Schiestl FP, Čurn V, Suda J (2012) Minority cytotypes in European populations of the Gymnadenia conopsea complex (Orchidaceae) greatly increase intraspecific and intrapopulation diversity. Ann Bot (Oxford) 110:977–986
Trávníček P, Kubátová B, Čurn V, Rauchová J, Krajníková E, Jersáková J, Suda J (2011) Remarkable coexistence of multiple cytotypes of the Gymnadenia conopsea aggregate (the fragrant orchid): evidence from flow cytometry. Ann Bot (Oxford) 107:77–87
Trávníček P, Ponert J, Urfus T, Jersáková J, Vrána J, Hřibová E, Doležel J, Suda J (2015) Challenges of flow-cytometric estimation of nuclear genome size in Orchids, a plant group with both whole-genome and progressively partial endoreplication. Cytometry A 87:958–966
Turland NJ, Wiersema JH, Barrie FR, Greuter W, Hawksworth DL, Herendeen PS, Knapp S, Kusber WH, Li DZ, Marhold K, May TW, McNeill J, Monro AM, Prado J, Price MJ, Smith GF (eds) (2018) International Code of Nomenclature for algae, fungi, and plants (Shenzhen Code) adopted by the Nineteenth International Botanical Congress Shenzhen, China, July 2017. Regnum Vegetabile 159, Koeltz Botanical Books, Glashütten
Tyteca D, Gathoye JL (2003) Morphometric analyses of the Dactylorhiza maculata (L) Soó group in western Europe. Ber Arbeitskreis Heimische Orchid 20:1–32
Vaucher C (1966) Contribution a l’étude cytologique du genre Dactylorchis (Klinge) Vermeulen. Bull Soc Neuchâtel Sci Nat 89:75–85
Vermeulen P (1947) Studies on Dactylorchids. Drukkerij Fa Schotanus & Jens, Utrecht
Vermeulen P (1968) Dactylorchis maculata und ihre Formen. Jahresber Naturwiss Vereins Wuppertal 21–22:68–76
Vlačiha V, Dundr R (2002) Mapování orchidejí v severozápadní části Čech v roce 2001 [Mapping of the orchids in northwestern Bohemia in 2001]. Roezliana 31:48–49 [in Czech]
Vlčko J, Dítě D, Kolník M (2003) Vstavačovité Slovenska [Orchids of Slovakia]. ZO SZOPK Orchidea, Zvolen [in Slovak]
Vojtěchová K, Kobrlová L, Schönswetter P, Duchoslav M (2023) Disentangling the taxonomic structure of the Allium paniculatum species complex in central and eastern Europe using molecular, cytogenetic and morphological tools. Preslia 95:119–163
Vöth W (1978) Biometrische Untersuchungen an Dactylorhiza maculata s. l. – Sippen in Niederösterreich (Orchidaceae). Linzer Biol Beitr 10:179–215
Vöth W, Greilhuber J (1980) Zur Karyosystematik von Dactylorhiza maculata s. l. und ihrer Verbreitung, insbesondere in Niederösterreich. Linzer Biol Beitr 12:415–468
Weiss H, Stuessy TF, Grau J, Baeza CM (2003) Chromosome reports from South American Hypochaeris (Asteraceae). Ann Missouri Bot Gard 90:56–63
Wild J, Kaplan Z, Danihelka J, Petřík P, Chytrý M, Novotný P, Rohn M, Šulc V, Brůna J, Chobot K, Ekrt L, Holubová D, Knollová I, Kocián P, Štech M, Štěpánek J, Zouhar V (2019) Plant distribution data for the Czech Republic integrated in the Pladias database. Preslia 91:1–24
Zarzycki K, Szeląg Z (2006) Red list of the vascular plants in Poland. W. Szafer Institute of Botany, Polish Academy of Sciences, Kraków
Acknowledgements
We are indebted to a number of botanists that accompanied us in the field, helped us with searching localities or collected samples for us. We are also grateful for technical assistance in the FCM labs operated by M. Jandová and J. Vrána. Valuable consultations were kindly provided by P. Trávníček (FCM of orchids), A. Sennikov and J. Danihelka (taxonomic nomenclature). Our thanks also go to M. Hedrén and one anonymous reviewer, as well as to the handling editor P. Koutecký, who significantly contributed to the improvement of this paper. The holotype voucher was prepared and scanned by M. Oulehlová. The respective authorities in nature conservation are acknowledged for granting us with the necessary permits.
Funding
Open access publishing supported by the National Technical Library in Prague. The research reported here was supported by an internal grant of Palacký University Olomouc (IGA_PrF_2023_001) and by the Austrian Federal Ministry of Education, Science and Research (BMBWF) within the programme Aktion CZ-AT, administrated by OeAD-GmbH. The article appears through the institutional support of long-term conceptual development of research institutions provided by the Ministry of Culture of the Czech Republic (ref. MK000094862).
Author information
Authors and Affiliations
Contributions
VT, PB, FL and BT conducted the field work and collected morphometric data. VT, MH, FL, EMT and HWS participated in the estimation of genome sizes and ploidy levels. MD and MH designed and performed the statistical analyses. MD analysed the ecological and environmental data. VT and BT carried out the red list categorization. VT drafted the manuscript with significant contributions of MD, MH and BT. All authors commented on and approved the manuscript. BT supervised the project.
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Taraška, V., Duchoslav, M., Hroneš, M. et al. Dactylorhiza maculata agg. (Orchidaceae) in Central Europe: Intricate Patterns in Morphological Variability, Cytotype Diversity and Ecology Support the Single-Species Concept. Folia Geobot 58, 151–188 (2023). https://doi.org/10.1007/s12224-024-09441-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12224-024-09441-0