Abstract
Ulvan is a sulfated heteropolysaccharide present in the cell wall of Ulva species with a unique structure and biological potential used in various fields. Chemical characterization was carried out to determine the structure of ulvan from Ulva fasciata Delile collected from Eastern Harbor, Alexandria coast, Egypt. Ulva contains 31.5% carbohydrate with a total ulvan content of 43.66% of total carbohydrate (13.75 g/100 g DW) and sulfate content of 20.45% of ulvan. FTIR spectrum presented signals of the sulfate ester (C–O–S) and sulfate groups (S=O), typical for ulvan. GC–MS revealed that ulvan was mainly composed of rhamnose and fucose. 1H-NMR spectra of ulvan showed identical behavior of monosaccharides nature with peaks characteristic of sulfated polysaccharides at 3.2–5.3 ppm region. Scanning electron micrographs (SEM) demonstrated amorphous architecture, and the sulfated nature of polysaccharides was emphasized by EDX analysis. The extracted ulvan showed significant antimicrobial activity against human and fish pathogens as well as antifouling bacteria with minimum inhibitory concentrations (MIC) of 8 µg/mL. The extracted ulvan exhibited potent antioxidant activity with a scavenging effect of 84.93% for 2,2-diphenyl-1-picrylhydrazy free radical (DPPH). Moreover, it showed anti-arthritic properties for the first time with a maximum inhibition of 86.04% with IC50 of 43.21%, indicating their potential value for the health and food industry.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
The field of functional food is currently attracting the attention of researchers, scientists, governments, politicians, and numerous global agencies to increase public understanding and use of these food items owing to their established health-promoting effects and low-toxicity concerns. Marine algae have been considered promising resources for new bioactive compounds in addition to their nutritional significance. It has been suggested that bioactive substances derived from marine species, such as algae, are more biologically effective than those derived from terrestrial sources (Safafar et al. 2015; El-Zokm et al. 2021). This is attributed to the higher bacterial populations in seawater than in air and the intense battle among marine algae and bacteria to survive in such an environment (Bixler and Bhushan 2012; Shannon and Abu-Ghannam 2016). In addition, algal biomass is naturally grown and collected from coastal regions in the sea and consider a sustainable, renewable and costless material that can be used as a source of valuable chemicals and biopolymers (Barakat et al. 2021; Hassan et al. 2021).
Pigments, phycobiliproteins, fatty acids, proteins, polysaccharides, vitamins, phenols and sterols are among the bioactive compounds produced by marine algae. These bioactive compounds have several applications, including antioxidant, antimicrobial (Elshobary et al. 2020; Osman et al. 2012), aquaculture (El‐Khodary et al. 2020; Zaki et al. 2021; El-Sayed et al. 2022), anti-inflammatory (Sarithakumari et al. 2013), anticancer activities (Chen et al. 2019), plant biostimulants (Ashour et al. 2021; Hassan et al. 2021), immunostimulatory (Tabarsa et al. 2018) and biofuel (Abomohra and Elshobary, 2019; Huo et al. 2020; El Shafay et al. 2021; Elshobary et al. 2021). Polysaccharides are intriguing materials for creating innovative systems for bioapplications, such as drug delivery and tissue engineering, as they have multiple functionalities in their structures and have interesting physicochemical properties and considerable biological activity (Shelke et al. 2014; Tziveleka et al. 2019). Marine seaweeds produce large quantities of indigestible sulfated polysaccharides, such as agar, alginate, carrageenan, ulvan, fucoidan, laminarin, etc., that are considered safer and non-immunogenic (Venkatesan et al. 2016; El-Seesy et al. 2021; El-Sayed et al. 2021; Ismail and Amer 2021), which have been employed in the food industry and are utilized as colloidal materials for improving the rheological characteristics of food products (Yang et al. 2020).
Among the marine flora encountered in Egypt, some macroalgae can become invasive or proliferative and have profound adverse ecological impacts (Shabaka 2018; Barakat et al. 2021). Over the past few years, seaweeds have been observed in the Eastern Harbor, Alexandria, Egypt. Seaweeds are driven to the shore by local wind and tides, resulting in economic losses and the damage of coastal marine habitats. Among these seaweeds, green algae species are diverse and are valuable components of the human diet in many countries worldwide. Specifically, the genus Ulva (sea lettuce) is rich in carbohydrates, vitamins, essential amino acids, minerals and soluble dietary fiber when eaten fresh or cooked (Pereira 2011; Ismail and Mohamed 2017; El-Zokm et al. 2021). Ulvan is one of the main cell wall sulfated polysaccharides that contributes from 9 to 36% dry weight of the biomass of common seaweeds of Ulvaceae genera. Its carbohydrate structure is complex and diverse, with the main constituents being rhamnose, glucuronic and iduronic acids, as well as xylose (Kidgell et al. 2019). The incorporation of rhamnose, a rare sugar found in bacteria and plants, and its sulfate content and uronic acids, distinguishe ulvan from other algal sulfated polysaccharides, such as fucoid and carrageenans (Alves et al. 2013). These distinct structural properties, combined with the wide range of biological activities and tunable physicochemical properties, have recently received much interest to ulvan as a promising material for therapeutic applications, such as antioxidant, anticoagulant, immunomodulating, antifouling and antihyperlipidemic capabilities (Mo’o et al. 2020).
Observably, most of the biological properties of sulfated polysaccharides such as ulvan were attributed to the presence of sulfate groups (Liang et al. 2014; Ismail and Amer 2021). Occasionally, the chemical properties, structure, molecular weight and sulfate amount of such polysaccharides vary with algal species, and consequently, their biological activity; hence, their chemical characterization is significantly required (Fernando et al. 2017).
The difficulty in regulating the structural composition of extracted polysaccharides is one downside of ulvan. Indeed, the structural composition of ulvan is influenced by a number of factors, including the algal species' origin and harvest season. Thus, the current work was suggested to extract and fully characterize ulvan from the green macroalgae Ulva fasciata Delile and then examined as antioxidant and antimicrobial agents against pathogenic and antifouling bacteria, which are particularly problematic and more resistant to antibiotics and biocides as well as its potential as an anti-arthritic activity that was investigated for the first time.
2 Materials and methods
2.1 Sampling and identification of the green alga
Green alga was handpicked during spring 2020 from the submerged rocks and substrates in the Eastern Harbor shallow water, Alexandria, Egypt. The collected green alga was identified morphologically according to taxonomical reference guides (Aleem 1993) and confirmed with Algae Base website (Guiry and Guiry 2020). The alga was rinsed with tap water to remove impurities and then dried to a constant weight on absorbent paper at room temperature (25 ± 2 °C). The dry sample was crushed and electrically ground into a powder form and then stored in paper bags in dry places until further use.
2.2 Chemical composition of U. fasciata Delile
Humidity was considered as the mass loss from a sample (1 g) after drying at 100 ± 2 °C (Elektro Helios oven J84234-2, 200 °C, Stockholm, Sweden). The residual mass in this sample was heated at 600 ± 10 °C (Fornitec 0159, 1200 °C, São Paulo, Brazil) to obtain ash (Pádua et al. 2004). The crude protein content was estimated using the Kjeldahl method using the distiller-digester (Procion 110 V, Brazil) (Yokoyama and Guimarães 1975). For lipid estimation, five grams of the sample were extracted with ethyl ether in Soxhlet for 5 h. The solvent was evaporated, and the residual lipids were measured gravimetrically (Pádua et al. 2004). Fibers were estimated by digesting five grams of the sample with 200 mL of 5% HCl for 30 min. The homogenate was filtrated and washed with hot water. The residue was digested again with 200 mL of 5% NaOH under reflux for 30 min. The homogenate was filtered and washed with water until negative alkaline reaction via methyl red indicative paper. The material was washed with 20 mL of ethyl alcohol followed by 20 mL of ethyl ether. The residue was dried at 100 °C for 2 h, and the residual fibers were calculated using the following equations, and the results were expressed as percentage odf dried seaweed (Pádua et al. 2004).
where WR and WS were the weight of the collected residual and dried seaweed (5 g), respectively.
Carbohydrate was calculated using the phenol–sulfuric acid method (Masuko et al. 2005) modified by Elshobary et al. (2015).
2.3 Ulvan extraction
Twenty grams of seaweed powder were rinsed with 200 mL, 80% cold ethanol under continuous stirring overnight at room temperature (25 ± 2 °C) to remove phenolic, free sugars, amino acids, lipidic, and colored compounds. The mixture was centrifuged at 10 °C and 5000×g for 10 min (Heraeus, Labofuge200, Thermo Scientific, United States) and the supernatant was removed to obtain defatted algal biomass. The residue was treated with hexane to confirm the removal of colored compounds, and the colorless residue was dried at room temperature in the fume hood. The depigmented powder was mixed with distilled water (1: 20 W/V) and extracted at 85–90 °C in a closed reflux system for 20 h with continuous stirring (Yamamoto 1980). The supernatants were collected by centrifugation for 10 min at 10°C and 8000×g. This process was repeated twice, and the supernatants were combined and concentrated by evaporation under reduced pressure at 60°C. The water extract was centrifuged, filtered and precipitated using 4 vol. of ethanol (99%) and kept at 4°C overnight. The precipitated polysaccharides were collected after centrifugation at 10°C and 8000×g for 10 min. The polysaccharides were washed and dehydrated with 99% ethanol and then dried at room temperature. To obtain highly purified ulvan, the dried polysaccharide was redissolved in distilled water, dialyzed against distilled water, and freeze-dried. The ulvan yield was calculated gravimetrically relative to the depigmented residue (Tako et al. 2015; Tabarsa et al. 2018).
2.4 Chemical composition of ulvan
2.4.1 Sulfated content
Total sulfate content within the ulvan was estimated following the modified BaCl2 turbidimetric method (Kolmert et al. 2000). Fifty milligrams of ulvan was hydrolyzed with 3 mL of 1 N HCl at 105 °C for 2 h, and the solution was completed to 50 mL with distilled water. The hydrolyzed polysaccharide was added to 2.5 mL of conditioning reagent (conc. HCl, 95% ethanol, glycerol, water [3:0.3:1:3] with 0.75% NaCl) shaken for 1 min at room temperature. Then, 5 mL of BaCl2 solution was added to the conditioned sample, stirred for 2 min with a magnetic stirrer, and the absorbance was recorded at 420 nm immediately (Schimatzu® UV-1800, Japan). The total sulfate content was expressed as a percentage based on a sodium sulfate standard.
2.4.2 UV–visible spectroscopy analysis
UV–VIS was used to demonstrate the success of the Ulvan polysaccharide purification step. At room temperature, 1 mg/mL of extracted ulvan was scanned with a UV–vis spectrophotometer in the 200 nm to 800 nm wavelength.
2.4.3 Infrared spectrum of ulvan from U. fasciata Delile
A Fourier-transform infrared spectrophotometer (FT-IR) was used to measure the polysaccharides' FT-IR spectrum (FTS-3000; Bio-Rad Laboratories Inc., CA, USA). The polysaccharide sample was mashed in a mortar with KBr powder (which had been baked in an oven at 100 °C to remove the water) and then pressed against a transparent sheet with a tablet press for FT-IR analysis in the frequency range of 4000–400 cm−1.
2.4.4 Determination of monosaccharides composition by GC–MS
Identification of the monosaccharides in the extracted ulvan was done by converting them into sugar derivatives before GC–MS analysis. About 20 mg of extracted ulvan was hydrolyzed using 2 mol/L sulfuric acid at 105 °C for 10 h. After cooling, the hydrolysate was neutralized with barium carbonate to pH 7.0. The residue was discarded after centrifugation, while the supernatant was filtered through a 0.20 μm syringe filter, lyophilized, then the dried sample was dissolved in 50 μL methanol. A silyating mixture of pyridine–hexamethyldisilazane–trimethyl chlorosilane (9:3:1 v/v/v) was mixed with 50 μL per mg of dried sample. About 2 μL from the methyl–silyl sugar derivatives was injected into GC–MS (MassHunter GC–MS 1989–2014, Agilent Technologies, Inc, NRC-Egypt). The separation and detection of the formed silyl sugar derivatives were accomplished using a previously reported method: the column used was HP5MS (30 m × 0.25 mm × 0.25 μm), detector and injector temperatures were set at 320°C, column temperature was first set at 100 °C for 1 min, then increased from 100 to 260 °C at 4 °C for 1 min, then the temperature was set for 10 min at 260 °C. Helium was used as carrier gas at 1 mL/min. The detected masses were identified by comparing with the standard masses in NIST-2014 library (Amornrut et al. 1999).
2.4.5 1H-nuclear magnetic resonance (NMR) spectroscopy
The spectra of 1H-NMR were recorded on a JEOL Ltd, Japan 500 FT-NMR spectrometer at 500.00 and 125.65 MHz, respectively. The polysaccharide (2%, W/V) was dissolved in D2O and measured at 37 or 60℃. The chemical shifts of 1H-NMR were expressed in parts per million (ppm) relative to sodium 3-(trimethylsilyl) propionic-2,2,3,3-d, acid (TSP, 0.00 ppm) as an internal standard.
2.4.6 Morphological and elemental studies of the extracted ulvan by SEM–EDX spectroscopy
Morphological and elemental studies were carried out using an Auriga field emission scanning electron microscope (SEM), a completely digital 30 kV Hi Resolution SEM connected with EDX and a range of backscattered (BS) and secondary detectors peculiar to the instrument. The X-ray spectra were stored through the latest from the Oxford offering: Oxford-Advanced AZtecEnergy package with an SDD 127 eV (Khan et al. 2008).
The sample was prepared in a Class II biosafety cabinet. Ag foil (Sigma Aldrich, 0.01 mm, 99.9% trace metals basis) was used to load the sample for SEM measurement. This conducting material was cut 1 cm × 1 cm and stacked with the SEM sample holder with double-sided tape. Numerous 10 µL spots of ulvan were poured onto Ag foil. The working distance and lens aperture sizes were 8 mm and 30 μm, respectively.
2.5 Antioxidant activity of ulvan
Antioxidant activity of the extracted ulvan was determined using two standard methods, 2,2-diphenyl-1-picrylhydrazyl (DPPH) free radical scavenging (Viturro et al. 1999; Hassan et al. 2021) and Hydrogen peroxide radical scavenging assay (Gülçin et al. 2004). These assays were performed using different concentrations (5, 10, 20, 30, 40 and 50 µg/mL) of extracted ulvan, and α-tocopherol was used as a positive control for the antioxidant activity. Concentrations of the most promising extract resulting in 50% inhibition of DPPH radicals (IC50) were calculated by GraphPad Prism 6 software.
2.6 Antimicrobial activity of ulvan from U. faciata Delile
The well-cut diffusion technique (Osman et al. 2012) was used to evaluate the antimicrobial activity of extracted ulvan against 11 bacterial pathogens (Bacillus subtlis ATCC 6633, Staphylococcus aureus ATCC 25923, Staphylococcus epidermids ATCC, Enterococcus faecalis ATCC 29219, and Listeria sp ATCC., Pseudomonas aeruginosa ATCC 9027, Klebsilla pneumonia ATCC, Escherichia coli ATCC 8739 and Bordetella sp. ATCC), three fungal reference strains (Aspergillus niger, Penicillum notatum, and Fusarum solani) and one yeast species (Candida albicans). A sterilized nutrient agar medium was used for bacteria and Sabouraud dextrose agar for fungi and yeast. The pre-cultured tested pathogen was mixed in agar media, poured into three sterile Petri dish plates, and allowed to solidify. About 100 µL of ulvan extract was transferred into each well of 0.7 cm and subjected to 4 °C incubation for 2 h, and then later incubated at 37 °C for 24–48 h. The results were obtained by measuring the diameter of the inhibition zone three times for each well and expressed in millimeters. Furthermore, the absolute unit (AU) was calculated according to Ibrahim (2012) as follow:
where Y is the diameter of the inhibition zone around the well, which its diameter is represented by X.
Moreover, antimicrobial activities of ulvan were recorded in the form of minimum inhibitory concentration (MIC) as recommended in the guide of standards of the CLSI (Clinical Laboratory Standardization Institute) (Schwalbe et al. 2007). MIC was determined, after incubation, by choosing the lowest inhibitory concentration of different ulvan concentrations according to the presence or absence of turbidity. The lower the MICs are, the higher the activity is.
2.7 In vitro anti-arthritic activity
0.05 mL of different concentrations of crude ulvan (0, 15, 30, 250, 500 and 1000 µg/mL) added to 0.45 mL of % W/V bovine serum albumin in aqueous solution and made up 0.5 mL of the reaction mixture. The pH was adjusted to 6.3 using 1 N HCl. The samples were heated for 3 min (57 °C) after incubating for 20 min at 37 °C. Allowed the sample to cool before adding 2.5 mL of phosphate buffer. At 416 nm, the turbidity generated was measured (Satoskar and Bhandarkar 2020). The percentage inhibition of protein denaturation was given by
Control indicates 100% protein denaturation. Diclofenac sodium was taken as standard.
2.8 Statistical analysis
The values of the different parameters were expressed as the mean (n = 3). The statistical analyses were performed by SPSS statistical software program (version 23.0). These data were subjected to the analysis of the variance (ANOVA) by applying the general linear model option (Duncan’s test) to determine significant differences among the samples at p < 0.05.
3 Results and discussion
3.1 Composition of U. fasciata Delile
The proximate chemical composition for the sample is summarized in Table 1. On a dry weight basis, carbohydrate 31.5% and ash 33.57% are the major contributors to the total biomass, followed by fiber 14.23% and protein 12.66%. This result agrees with that detected by El-Zokm et al. (2021) and Ismail and Mohamed (2017) for the same algal species. This result recommended this alga for use as a dietary supplement for humans and animals.
3.1.1 Characterization of the extracted ulvan
The total ulvan content after extraction relative to the U. fasciata dry weight was 43.66% of total carbohydrate (13.75 g/100 g DW). The sulfate content of ulvan was 20.45% of ulvan, reflecting the high content of the sulfate within the ulvan. In this context, ulvan is one of the major cell wall polysaccharides that contribute from 9 to 36% dry weight of the biomass of Ulva species (Lahaye and Robic 2007). There are three polymers besides Ulvan in the Ulva cell wall: cellulose, glucuronan, and xyloglucan. Collectively, they present with ulvan up to 45% of the dry weight biomass (Lahaye and Robic 2007). Ulvan isolated from numerous Ulvales species have sulfate contents ranged from 14.3 to 23.2% (Thanh et al. 2016; Tabarsa et al. 2018; Tziveleka et al. 2019).
3.2 UV spectrum analysis
UV–visible spectroscopy is utilized to evaluate chromophore groups of atoms with strongly absorbing electronic transitions. Figure 1 depicts the UV–visible absorption spectrum of the ulavn tested in this study. The findings revealed that maximum absorption was measured between 200 and 220 nm. The wavelength area of 200–220 nm is frequently caused by n−σ* and/or π−π* transitions, which are reported in several functional groups, such as carboxyl, carbonyl, and ester of the polysaccharides. The results show ulvan had no obvious characteristic absorption peak at 260–280 nm, verifying that the extracted ulvan did not contain nucleic acid or protein, respectively (Trabelsi et al. 2009; Huo et al. 2022).
3.3 Structural characterization of sulfated polysaccharides by FTIR
The FTIR analysis of extracts showed typical polysaccharide absorption peaks containing carboxyl and sulfate groups (Fig. 2). The spectra are identical and comparable to the IR spectra of the sulfated polysaccharides obtained from ulvan of different Ulva species in the literature (Tako et al. 2015; Thanh et al. 2016; Tabarsa et al. 2018; Chi et al. 2020a). The FTIR spectra of the ulvan showed a wide and strong absorption band at 3375–3380 cm−1 corresponds to the stretch vibration of O–H in the polysaccharide resulting from intermolecular and intramolecular hydrogen bonding (Robic et al. 2009), the weak peak (shoulder band) at 2925–2928 cm−1 is attributed to the stretch vibration of the aliphatic C–H of a methyl group and is characteristic of polysaccharides.
FTIR spectra of ulvans showed a maximum band at about 1076 cm−1, which refers to the C−O stretching of the main rhamnose sugar. The sulfated nature of the polysaccharide is ascertained by the absorption band in the region 820–856 cm−1 which is related to stretching of axial sulfate groups related to the C–O–S group, as well as by the shoulder at 1225–1226 cm−1 which is assigned (ascribed) to the stretching vibration of the sulfate ester (S=O) group as confirmed by Castro et al. (2006) for ulvan from U. rigida, U. pertusa (Tako et al. 2015), U. lactuca (Thanh et al. 2016) and U. clathrata (Chi et al. 2020b). This result confirms that the ulvan from U. fasciata contains more than one type of sulfate group. Also, the band at the region 748–750 cm−1 was reported as a typical signature to sugar cycles such as ulvans (Robic et al. 2009; Hernández-Garibay et al. 2011).
3.4 Monosaccharides composition via GC–MS
Ulvan is a polysaccharide whose heterogeneous composition varies according to the algal biomass, taxonomic origin and harvesting season (Lahaye and Robic 2007). The difficulty to accurately define the composition of ulvan may be attributed to the complexity of its structure and the presence of several types of sugars (Alves et al. 2013; Sari-Chmayssem et al. 2019). Acid hydrolysis is the most effective method of depolymerizing the polysaccharides into their monomeric units, followed by their identification using the appropriate chromatographic technique (Costa et al. 2012; Elshobary et al. 2016). The monosaccharide composition of the sulfated polysaccharides revealed that they might contain heterogeneous polymers due to the variation of monosaccharides. Furthermore, the polysaccharides also contain varying levels of sugars (Sari-Chmayssem et al. 2019).
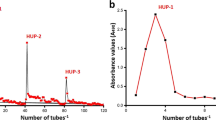
The results are depicted in Table 2 and Fig. 3, revealing the presence of various monosaccharides with different contents in the order: l-rhamnose (34.63%) > β-arabinopyranose (16.25%) > l-fucose (15.12%) > rhamnopyranose (8.16%) > fucopyranose (15.62%) > mannopyranose (9.95%), while the contents of α-d-glactopyranose is neglected. The sulfated polysaccharide from U. fasciata was mainly composed of rhamnose (42.79%) of l-rhamnose, and l-( +)-rhamnopyranose and fucose (30.47%) of l-fucose and β-l-(-)-fucopyranose as major compositions, while arabinose and mannose were in minor contents. The composition of ulvan from U. faciata was similar to those of ulvan from other studies that demonstrated rhamnose, the main sugar of ulvan, and its content ranged from 12.73 to 45% (Tabarsa et al. 2018; Van Tran et al. 2018). Ulvan of U. fasciata lacks uronic acid sugars, such as glucuronic or galacturonic acids, which may be due to the substitution of uronic acids sugars with sulfated sugar residues (Yaich et al. 2014; de Freitas et al. 2015; Thanh et al. 2016).
3.5 Structural analysis of the extracted ulvan by 1H NMR
Ulvan is a group of heteropolysaccharides composed mainly of rhamnose, xylose, glucose, and sulfate, with low contents of galactose, mannose, and arabinose (Yaich et al. 2017). It has been documented that ulvan possess complex and heterogeneous structures lacking regularity, and the existence of sulfate groups often interrupts the NMR determination of connectivity and branching. However, the assignment of most of the proton signals in the present investigation was based on the chemical shifts reported for ulvans in literature (Tako et al. 2015; Yaich et al. 2017; Van Tran et al. 2018). 1H-NMR spectrum for ulvan extract (Fig. 4) is comparable with previous data for ulvan. Thirteen chemical signals were observed as shown in Fig. 4. Signals at 5.43 and 4.79 ppm were assigned to be α-l-idulonic acid and sulfated α-l-rhamnose, respectively (Hernández-Garibay et al. 2011; Tako et al. 2015). The two intense peaks at 1.20 and 1.33 are assigned to the protons of the methyl groups of non-sulfated and sulfated α-l-rhamnosyl residues, respectively (Nakamura et al. 2011; Tako et al. 2015). The region 3.37–4.13 ppm is probably representative of ring protons (Thanh et al. 2016), and the hybrid nature of polysaccharides, rich in rhamnose and the presence of xylose, glucose, uronic acids and galactose as observed by the overlapped and broadened signals. The sulfated nature of the α-l-rhamnose was emphasized by the signals at 1.31 and 5.43 ppm as reported for U. clathrata (Chi et al. 2020b).
It was a hurdle to assign all the protons bound to the –OH groups in the region of 4.5–5.4 ppm because the signals are mostly superimposed by the peak of the deuterium oxide (D2O; 4.78 ppm); however, the protons linked to other groups are allocated in the region of 3.4–3.6 ppm (Barcellos et al. 2018).
3.6 Morphological and elemental studies by SEM and EDX spectroscopy
The SEM micrographs demonstrated amorphous architecture (Fig. 5) with a high percentage of C, O, and S elements. Interestingly, the suggestion above of the sulfated nature of the polysaccharides was emphasized from the EDX analysis (Fig. 6; Table 3), which indicated that the presence of different elements, particularly the presence of some elements like C, N, O, S, Cl, Na, Ca, K, and Fe, ascertaining the existence of the sulfate group within the third order of mass % (10.56%) after oxygen (48.97%) and carbon (25.53%) (Table 3) in extracted ulvan as confirmed by the IR and 1HNMR analyses. The amorphous architecture of the ulvan may be related to the presence of calcium-bound that was difficult to remove with ethanol precipitation method. This influences the particulate shape and aggregation in the SEM micrograph.
3.7 Antioxidant activity of the extracted ulvan
Several polysaccharides produced from seaweeds have shown superior antioxidant activities, and they could be used as prospective candidates for safe, stabilized, and efficacious natural antioxidants owing to their low toxicity and lesser side effects, which is a major issue with synthetic antioxidants (Kumar et al. 2008; Casas-Arrojo et al. 2021). The antioxidant activity of polysaccharides is dependent on different factors, including the type, purity, molecular weight, structure, sidechain, and attached groups (Sun et al. 2014; Ismail and Amer 2021). In the concentration range of 0–50 µg/mL, the scavenging rate of H2O2 and DPPH free radicals increased significantly (ANOVA, p = 0.012 and 0.006, respectively) by increasing the concentration of ulvan. Ulvan as sulfate polysaccharide has a promising in vitro antioxidant property, with an inhibitory effect of 84.93% on DPPH and 55.5% on superoxide radicals compared to the standard α-tocopherol of 83.91 and 61.32%, respectively (Fig. 7). Yaich et al. (2017) reported that scavenging activity against DPPH of ulvan from U. lactuca was 75.0.2% at 400 μg/mL. In another study, ulvan from U. intestinalis showed 56.18% DPPH scavenger activity (Peasura et al. 2015). Compared to these results, the current ulvan exhibited greater scavenging activity toward DPPH radical than that recorded in U. lactuca (Yaich et al. 2017) or U. intestinalis (Peasura et al. 2015). Ulvan extracted from U. fasciata exhibited the greatest scavenging activity (IC50 = 25.26 μg/mL) toward DPPH radical less than α-tocopherol standard (IC50 = 25.91 μg/mL), which reflect its efficiency as antioxidant activity.
According to some studies, the key factors affecting the radical scavenging capacity of polysaccharides from marine algae are sulfate content, monosaccharide composition, and molecular weight (Li et al. 2013; Yaich et al. 2017). It was proposed that the sulfate group could activate the hydrogen atom of the anomeric carbon, thereby contributing to the polysaccharide's hydrogen-donating ability (Wang et al. 2010; Peasura et al. 2015). The high sulfate content of extracted ulvan (20%) could explain the higher recorded radical scavenging activity.
3.8 Antimicrobial activity of extracted ulvan
Clearly, the extracted ulvan exhibited antimicrobial activity against some Gram-positive and Gram-negative bacteria besides yeast C. albicans. Most of them are known as pathogenic and multi-resistant antifouling bacteria, such as the Gram-positive E. faecalis, Staph. aureus and Staph. epidermidis, and the Gram-negative E. coli, K. pneumoniae and P. aeruginosa (Donlan 2001; Shunmugaperumal 2010). The data presented in Table 4 showed that many bacterial pathogens were influenced by ulvan in low concentrations with AU ranging from 1.7 to 2.3; however, B. subtilis Staph. aureus, Listeria sp., and Bordetella sp. were inhibited in high concentrations of more than 1000 µg/mL. The MIC values ranged between 32 µg/mL against E. coli to 16 µg/mL against E. faecalis, and 8 µg/mL C. albicans. Moreover, weak suppression was detected against the tested fungal pathogens (A. niger, P. notatum and F. solani), even though many studies on the bioactivities of ulvan, such as antioxidant, anticoagulant, immunomodulatory, and anticancer activity, just a few studies on antimicrobial activities have been published (Van Tran et al. 2018). The sulfated polysaccharide extract of U. armoricana showed antibacterial activity against 42 Gram-positive and Gram-negative bacterial strains (Berri et al. 2016). However, the MIC of the most sensitive species (160–6250 µg/mL) was relatively higher than recorded in the current study. Ulvan isolated from the genus U. reticulata was efficacious against E. coli, P. aeruginosa and E. cloace (Van Tran et al. 2018). Differential antibacterial activity of ulvan could be due to their branching architectures. However, aspects impacting ulvan's antibacterial action, such as molecular weight, the density of charged groups, and shape, must be investigated further. Noteworthy, the antibacterial activity of ulvan was primarily demonstrated by its ability to prevent the formation of biofilms on coated surfaces (Gadenne et al. 2013; Junter et al. 2016).
3.9 In vitro anti-arthritic activity
Denaturation of proteins and production of autoantigens is the main cause of rheumatoid arthritis (Shravan et al. 2011). Accordingly, inhibition of protein denaturation was examined using the extracted ulvan that showed potent inhibition of protein denaturation very close to the diclofenac sodium standard. In the concentration range of 0–1000 µg/mL, the anti-arthritic activity increased significantly (ANOVA, p = 0.013) by increasing the concentration of ulvan (Fig. 8). The maximum inhibition (86.04%) was recorded at 1 mg/mL with IC50 of 43.21 µg/mL, which was insignificantly different from the diclofenac sodium standard. In this context, many studies have shown sulfated polysaccharides extracted from different seaweeds, such as Gelidium pacificum (Cui et al. 2019); Corallina officinalis and Pterocladia capillacea (Ismail and Amer 2021); Ulva ohnoi (Kidgell et al. 2020); Ulva armoricana (Berri et al. 2017), have anti-inflammatory properties that may be due to their inhibitory effects on the production of pro-inflammatory mediators (Berri et al. 2017; Kidgell et al. 2020). Based on these findings, Ulvan of U. fasciata is able to control the production of autoantigens and, as a result, inhibit protein denaturation.
4 Conclusion
It could be concluded that the use of the marine ulvan, sulfated heteropolysaccharide, from Ulva fasciata Delile has many potential biological activities which could be applied in both the biomedical field and food industry. First, chemical characterization was studied to assure the extracted ulvan structure using FTIR, GC–MS and 1HNMR analysis and confirmed by SEM and EDX analysis to show the sulfated nature of polysaccharides due to the FTIR bands of the sulfate ester and sulfate groups, typical for ulvan. The extracted ulvan exhibited potent antioxidant activity reaching 84.93 and 55.5% against DPPH and superoxide radical scavenging, respectively. In addition, ulvan showed antimicrobial activity against microfoulers or/and pathogenic bacteria and fungi with MIC of 8 µg/mL against K. pneumonia and C. albican, as well as anti-arthritic properties up to 86.04%. These results indicate that the extracted ulvan has a potential value for health and food applications.
References
Abomohra AE, Elshobary ME (2019) Biodiesel, bioethanol and biobutanol production from microalgae biomass. In: Alam MA, Wang Z (eds) Microalgae biotechnology for development of biofuel and waste water treatment. Springer Singapore Press, Singapore, pp 293–321. https://doi.org/10.1007/978-981-13-2264-8
Aleem AA (1993) The marine Algae of Alexandria, Egypt (139 p.). Privately published, Alexandria: 1–55.
Alves A, Sousa RA, Reis RL (2013) A practical perspective on ulvan extracted from green algae. J Appl Phycol 25:407–424. https://doi.org/10.1007/s10811-012-9875-4
Amornrut C, Toida T, Imanari T, Woo E-R, Park H, Linhardt R, Wu SJ, Kim YS (1999) A new sulfated β-galactan from clams with anti-HIV activity. Carbohyd Res 321:121–127. https://doi.org/10.1016/S0008-6215(99)00188-3
Ashour M, Hassan SM, Elshobary ME, Ammar GAG, Gaber A, Alsanie WF, Mansour AT, El-Shenody R (2021) Impact of commercial seaweed liquid extract (TAM®) biostimulant and its bioactive molecules on growth and antioxidant activities of hot pepper (Capsicum annuum). Plants 10:1045. https://doi.org/10.3390/plants10061045
Barakat KM, El-Sayed HS, Khairy HM, El-Sheikh MA, Al-Rashed SA, Arif IA, Elshobary ME (2021) Effects of ocean acidification on the growth and biochemical composition of a green alga (Ulva fasciata) and its associated microbiota. Saudi J Biol Sci 28:5106–5114. https://doi.org/10.1016/j.sjbs.2021.05.029
Barcellos PG, Rodrigues JAG, de Queiroz INL, de Araújo IWF, Benevides NMB, de Souza Mourão PA (2018) Structural and physical-chemical analyses of sulfated polysaccharides from the sea lettuce Ulva lactuca and their effects on thrombin generation. Acta Sci Biol Sci 40:e34916–e34916
Berri M, Slugocki C, Olivier M, Helloin E, Jacques I, Salmon H, Demais H, Le Goff M, Collen PN (2016) Marine-sulfated polysaccharides extract of Ulva armoricana green algae exhibits an antimicrobial activity and stimulates cytokine expression by intestinal epithelial cells. J Appl Phycol 28:2999–3008. https://doi.org/10.1007/s10811-016-0822-7
Berri M, Olivier M, Holbert S, Dupont J, Demais H, Le Goff M, Collen PN (2017) Ulvan from Ulva armoricana (Chlorophyta) activates the PI3K/Akt signalling pathway via TLR4 to induce intestinal cytokine production. Algal Res 28:39–47
Bixler GD, Bhushan B (2012) Biofouling: lessons from nature. Philos Trans R Soc a: Math Phys Eng Sci 370:2381–2417
Casas-Arrojo V, Decara J, de Los Ángeles Arrojo-Agudo M, Pérez-Manríquez C, Abdala-Díaz RT (2021) Immunomodulatory, antioxidant activity and cytotoxic effect of sulfated polysaccharides from Porphyridium cruentum. (s.f.gray) nägeli. Biomolecules. https://doi.org/10.3390/biom11040488
Castro R, Piazzon MC, Zarra I, Leiro J, Noya M, Lamas J (2006) Stimulation of turbot phagocytes by Ulva rigida C. Agardh Polysaccharides. Aquaculture 254:9–20
Chen X, Song L, Wang H, Liu S, Yu H, Wang X, Li R, Liu T, Li P (2019) Partial characterization, the immune modulation and anticancer activities of sulfated polysaccharides from filamentous microalgae Tribonema sp. Molecules 24:1–11. https://doi.org/10.3390/molecules24020322
Chi Y, Zhang M, Wang X, Fu X, Guan H, Wang P (2020)a Ulvan lyase assisted structural characterization of ulvan from Ulva pertusa and its antiviral activity against vesicular stomatitis virus. Int J Biol Macromol 157:75–82. https://doi.org/10.1016/j.ijbiomac.2020.04.187
Chi Y, Li H, Wang P, Du C, Ye H, Zuo S, Guan H, Wang P (2020)b Structural characterization of ulvan extracted from Ulva clathrata assisted by an ulvan lyase. Carbohyd Polym 229:115497. https://doi.org/10.1016/j.carbpol.2019.115497
Costa C, Alves A, Pinto PR, Sousa RA, da Silva EAB, Reis RL, Rodrigues AE (2012) Characterization of ulvan extracts to assess the effect of different steps in the extraction procedure. Carbohyd Polym 88:537–546
Cui M, Wu J, Wang S, Shu H, Zhang M, Liu K, Liu K (2019) Characterization and anti-inflammatory effects of sulfated polysaccharide from the red seaweed Gelidium pacificum Okamura. Int J Biol Macromol 129:377–385
de Freitas MB, Ferreira LG, Hawerroth C, Duarte MER, Noseda MD, Stadnik MJ (2015) Ulvans induce resistance against plant pathogenic fungi independently of their sulfation degree. Carbohyd Polym 133:384–390
de Pádua M, Fontoura PSG, Mathias AL (2004) Chemical composition of Ulvaria oxysperma (Kützing) bliding, Ulva lactuca (Linnaeus) and Ulva fascita (Delile). Braz Arch Biol Technol 47:49–55
Donlan RM (2001) Biofilms and device-associated infections. Emerg Infect Dis 7:277
EL-Seesy AI, Elshobary ME, He Z (2021) Biofuel versus fossil fuel. In: Handbook of Algal biofuels Aspects of Cultivation, Conversion, and Biorefinery, 181–193. Elsevier. https://doi.org/10.1016/B978-0-12-823764-9.00027-3
El Shafay SM, Gaber A, Alsanie WF, Elshobary ME (2021) Influence of nutrient manipulation on growth and biochemical constituent in Anabaena variabilis and Nostoc muscorum to enhance biodiesel production. Sustainability (switzerland) 13:9081. https://doi.org/10.3390/su13169081
El-Khodary GM, El-Sayed HS, Khairy HM, El-Sheikh MA, Qi X, Elshobary ME (2020) Comparative study on growth, survival and pigmentation of Solea aegyptiaca larvae by using four different microalgal species with emphasize on water quality and nutritional value. Aquacult Nutr. https://doi.org/10.1111/anu.13211
El-Sayed AAM, Abouzeid FM, Ismail MM, ElZokm GM (2021) Characterization and utilization of Sargassum linifolium and Stypopodium schimperi polysaccharides as blue inhibitors for steel electo-polishing. Water Sci Technol 83:409–424
El-Sayed HS, Elshobary ME, Barakat KM, Khairy HM, El-Sheikh MA, Czaja R, Allam B, Senousy HH (2022) Ocean acidification induced changes in Ulva fasciata biochemistry may improve Dicentrarchus labrax aquaculture via enhanced antimicrobial activity. Aquaculture 560:738474. https://doi.org/10.1016/j.aquaculture.2022.738474
Elshobary ME, Osman MEH, Abushady AM, Piercey-Normore MD (2015) Comparison of lichen-forming cyanobacterial and green algal photobionts with free-living algae. Cryptogamie Algol 36:81–100. https://doi.org/10.7872/crya.v36.iss1.2015.81
Elshobary ME, Osman ME, Abo-Shady AM, Komatsu E, Perreault H, Sorensen J, Piercey-Normore MD (2016) Algal carbohydrates affect polyketide synthesis of the lichen-forming fungus Cladonia rangiferina. Mycologia 108:646–656. https://doi.org/10.3852/15-263
Elshobary ME, El-Shenody RA, Ashour M, Zabed HM, Qi X (2020) Antimicrobial and antioxidant characterization of bioactive components from Chlorococcum minutum. Food Biosci 35:100567. https://doi.org/10.1016/j.fbio.2020.100567
Elshobary ME, El-Shenody RA, Abomohra AE (2021) Sequential biofuel production from seaweeds enhances the energy recovery: A case study for biodiesel and bioethanol production. Int J Energy Res 45:6457–6467. https://doi.org/10.1002/er.6181
El-Zokm GM, Ismail MM, El-Said GF (2021) Halogen content relative to the chemical and biochemical composition of fifteen marine macro and micro algae: nutritional value, energy supply, antioxidant potency, and health risk assessment. Environ Sci Pollut Res 28:14893–14908
Fernando IPS, Sanjeewa KKA, Samarakoon KW, Lee WW, Kim H-S, Kang N, Ranasinghe P, Lee H-S, Jeon Y-J (2017) A fucoidan fraction purified from Chnoospora minima; a potential inhibitor of LPS-induced inflammatory responses. Int J Biol Macromol 104:1185–1193
Gadenne V, Lebrun L, Jouenne T, Thebault P (2013) Antiadhesive activity of ulvan polysaccharides covalently immobilized onto titanium surface. Colloids Surf B 112:229–236. https://doi.org/10.1016/j.colsurfb.2013.07.061
Guiry MD, Guiry G (2020) AlgaeBase. World-wide electronic publication, National university of Ireland, Galway. http://www.algaebase.org/
Gülçin İ, Mshvildadze V, Gepdiremen A, Elias R (2004) Antioxidant activity of saponins isolated from ivy: α-hederin, hederasaponin-C, hederacolchiside-E and hederacolchiside-F. Planta Med 70:561–563
Hassan SM, Ashour M, Soliman AAF, Hassanien HA, Alsanie WF, Gaber A, Elshobary ME (2021) The potential of a new commercial seaweed extract in stimulating morpho-agronomic and bioactive properties of Eruca vesicaria (L.) cav. Sustainability (switzerland) 13:4485. https://doi.org/10.3390/su13084485
Hernández-Garibay E, Zertuche-González JA, Pacheco-Ruíz I (2011) Isolation and chemical characterization of algal polysaccharides from the green seaweed Ulva clathrata (Roth) C. Agardh. J Appl Phycol 23:537–542. https://doi.org/10.1007/s10811-010-9629-0
Huo S, Basheer S, Liu F, Elshobary M, Zhang C, Qian J, Xu L, Arslan M, Cui F, Zan X, Zhu F, Zou B, Ding Q, Ma H (2020) Bacterial intervention on the growth, nutrient removal and lipid production of filamentous oleaginous microalgae Tribonema sp. Algal Res 52:102088. https://doi.org/10.1016/j.algal.2020.102088
Huo S, Wang H, Chen J, Hu X, Zan X, Zhang C, Qian J, Zhu F, Ma H, Elshobary M (2022) A preliminary study on polysaccharide extraction, purification, and antioxidant properties of sugar-rich filamentous microalgae Tribonema minus. J Appl Phycol. https://doi.org/10.1007/s10811-021-02630-w
Ibrahim HAH (2012) Antibacterial carotenoids of three Holothuria species in Hurghada, Egypt. Egypt J Aquat Res 38:185–194. https://doi.org/10.1016/j.ejar.2013.01.004
Ismail MM, Amer MS (2021) Characterization and biological properties of sulfated polysaccharides of Corallina officinalis and Pterocladia capillacea. Acta Bot Bras 34:623–632
Ismail MM, Mohamed SE (2017) Differentiation between some Ulva spp. by morphological, genetic and biochemical analyses. Baвилoвcкий Жypнaл Гeнeтики и Ceлeкции 21:360–367
Junter GA, Thébault P, Lebrun L (2016) Polysaccharide-based antibiofilm surfaces. Acta Biomater 30:13–25. https://doi.org/10.1016/j.actbio.2015.11.010
Khan AS, Ahmed Z, Edirisinghe MJ, Wong FSL, Rehman IU (2008) Preparation and characterization of a novel bioactive restorative composite based on covalently coupled polyurethane–nanohydroxyapatite fibres. Acta Biomater 4:1275–1287. https://doi.org/10.1016/j.actbio.2008.04.016
Kidgell JT, Magnusson M, de Nys R, Glasson CRKK (2019) Ulvan: A systematic review of extraction, composition and function. Algal Res 39:101422. https://doi.org/10.1016/j.algal.2019.101422
Kidgell JT, Glasson CRK, Magnusson M, Vamvounis G, Sims IM, Carnachan SM, Hinkley SFR, Lopata AL, de Nys R, Taki AC (2020) The molecular weight of ulvan affects the in vitro inflammatory response of a murine macrophage. Int J Biol Macromol 150:839–848. https://doi.org/10.1016/j.ijbiomac.2020.02.071
Kolmert Å, Wikström P, Hallberg KB (2000) A fast and simple turbidimetric method for the determination of sulfate in sulfate-reducing bacterial cultures. J Microbiol Methods 41:179–184
Kumar KS, Ganesan K, Rao PVS (2008) Antioxidant potential of solvent extracts of Kappaphycus alvarezii (Doty) Doty–An edible seaweed. Food Chem 107:289–295
Lahaye M, Robic A (2007) Structure and functional properties of ulvan, a polysaccharide from green seaweeds. Biomacromol 8:1765–1774
Li B, Liu S, Xing R, Li K, Li R, Qin Y, Wang X, Wei Z, Li P (2013) Degradation of sulfated polysaccharides from Enteromorpha prolifera and their antioxidant activities. Carbohyd Polym 92:1991–1996
Liang W, Mao X, Peng X, Tang S (2014) Effects of sulfate group in red seaweed polysaccharides on anticoagulant activity and cytotoxicity. Carbohyd Polym 101:776–785
Masuko T, Minami A, Iwasaki N, Majima T, Nishimura S-I, Lee YC (2005) Carbohydrate analysis by a phenol–sulfuric acid method in microplate format. Anal Biochem 339:69–72
Mo’o C, Ratu F, Wilar G, Devkota HP, Wathoni N (2020) Ulvan, a polysaccharide from macroalga Ulva sp.: a review of chemistry, biological activities and potential for food and biomedical applications. Appl Sci 10:5488
Nakamura M, Yamashiro Y, Konishi T, Hanasiro I, Tako M (2011) Structural characterization of rhamnan sulfate isolated from commercially cultured Monostroma nitidum (Hitoegusa). Nippon Shokuhin Kagaku Kogaku Kaishi 58:245–251
Osman MEH, Abu-Shady AM, Elshobary ME, Osman MEH, Abu-Shady AM, Elshobary ME (2012) The seasonal fluctuation of the antimicrobial activity of some macroalgae collected from Alexandria Coast, Egypt. Salmonella - distribution, adaptation, control measures and molecular technologies. InTech, Rijeka, pp 173–186
Peasura N, Laohakunjit N, Kerdchoechuen O, Wanlapa S (2015) Characteristics and antioxidant of Ulva intestinalis sulphated polysaccharides extracted with different solvents. Int J Biol Macromol 81:912–919. https://doi.org/10.1016/j.ijbiomac.2015.09.030
Pereira L (2011) A review of the nutrient composition of selected edible seaweeds. In: Pomin VH (ed) Seaweed: ecology, nutrient composition and medicinal uses. Nova Science Publishers, Inc, Coimbra, pp 15–47
Robic A, Bertrand D, Sassi J-F, Lerat Y, Lahaye M (2009) Determination of the chemical composition of ulvan, a cell wall polysaccharide from Ulva spp. (Ulvales, Chlorophyta) by FT-IR and chemometrics. J Appl Phycol 21:451–456. https://doi.org/10.1007/s10811-008-9390-9
Safafar H, Van WJ, Møller P, Jacobsen C (2015) Carotenoids, phenolic compounds and tocopherols contribute to the antioxidative properties of some microalgae species grown on industrial wastewater. Mar Drugs 13:7339–7356. https://doi.org/10.3390/md13127069
Sari-Chmayssem N, Taha S, Mawlawi H, Guégan J-P, Jeftić J, Benvegnu T (2019) Extracted Ulvans from green algae Ulva linza of Lebanese origin and amphiphilic derivatives: evaluation of their physico-chemical and rheological properties. J Appl Phycol 31:1931–1946
Sarithakumari CH, Renju GL, Kurup GM (2013) Anti-inflammatory and antioxidant potential of alginic acid isolated from the marine algae, Sargassum wightii on adjuvant-induced arthritic rats. Inflammopharmacology 21:261–268. https://doi.org/10.1007/s10787-012-0159-z
Satoskar RS, Bhandarkar SD (2020) Pharmacology and pharmacotherapeutics. Elsevier, New Delhi
Schwalbe R, Steele-Moore L, Goodwin AC (2007) Antimicrobial susceptibility testing protocols. CRC Press, New York
Shabaka SH (2018) Checklist of seaweeds and seagrasses of Egypt (Mediterranean Sea): A review. Egypt Jo Aquat Res 44:203–212. https://doi.org/10.1016/j.ejar.2018.08.001
Shannon E, Abu-Ghannam N (2016) Antibacterial derivatives of marine algae: An overview of pharmacological mechanisms and applications. Mar Drugs. https://doi.org/10.3390/md14040081
Shelke NB, James R, Laurencin CT, Kumbar SG (2014) Polysaccharide biomaterials for drug delivery and regenerative engineering. Polym Adv Technol 25:448–460. https://doi.org/10.1002/pat.3266
Shravan KN, Kishore G, Siva KG, Sindhu PES (2011) In vitro anti-inflammatory and anti-arthritic activity of leaves of Physalis angulata L. Int J Pharm Ind Res 1:211–213
Shunmugaperumal T (2010) Biofilm eradication and prevention: a pharmaceutical approach to medical device infections. Wiley, Hoboken
Sun Y, Wang H, Guo G, Pu Y, Yan B (2014) The isolation and antioxidant activity of polysaccharides from the marine microalgae Isochrysis galbana. Carbohyd Polym 113:22–31. https://doi.org/10.1016/j.carbpol.2014.06.058
Tabarsa M, You SG, Dabaghian EH, Surayot U (2018) Water-soluble polysaccharides from Ulva intestinalis: molecular properties, structural elucidation and immunomodulatory activities. J Food Drug Anal 26:599–608. https://doi.org/10.1016/j.jfda.2017.07.016
Tako M, Tamanaha M, Tamashiro Y, Uechi S (2015) Structure of Ulvan isolated from the edible green seaweed, Ulva pertusa. Adv Biosci Biotechnol 06:645–655. https://doi.org/10.4236/abb.2015.610068
Thanh TTT, Quach TMT, Nguyen TN, Vu Luong D, Bui ML, Van TTT, Luong DV, Bui ML, Van Tran TT, Vu Luong D, Bui ML, Van TTT (2016) Structure and cytotoxic activity of ulvan extracted from green seaweed Ulva lactuca. Int J Biol Macromol 93:695–702. https://doi.org/10.1016/j.ijbiomac.2016.09.040
Trabelsi L, M’sakni NH, Ben Ouada H, Bacha H, Roudesli S (2009) Partial characterization of extracellular polysaccharides produced by cyanobacterium Arthrospira platensis. Biotechnol Bioprocess Eng 14:27–31. https://doi.org/10.1007/s12257-008-0102-8
Tziveleka LA, Ioannou E, Roussis V (2019) Ulvan, a bioactive marine sulphated polysaccharide as a key constituent of hybrid biomaterials: a review. Carbohyd Polym 218:355–370. https://doi.org/10.1016/j.carbpol.2019.04.074
Van Tran TT, Truong HB, Tran NHV, Quach TMT, Nguyen TN, Bui ML, Yuguchi Y, Thanh TTT (2018) Structure, conformation in aqueous solution and antimicrobial activity of ulvan extracted from green seaweed Ulva reticulata. Nat Prod Res 32:2291–2296. https://doi.org/10.1080/14786419.2017.1408098
Venkatesan J, Anil S, Kim S-K, Shim M (2016) Seaweed polysaccharide-based nanoparticles: preparation and applications for drug delivery. Polymers 8:30. https://doi.org/10.3390/polym8020030
Viturro C, Molina A, Schmeda-Hirschmann G (1999) Free radical scavengers from Mutisia friesiana (asteraceae) and Sanicula graveolens (apiaceae). Phytother Res 13:422–424. https://doi.org/10.1002/(SICI)1099-1573(199908/09)13:5%3c422::AID-PTR462%3e3.0.CO;2-M
Wang J, Zhang Q, Zhang Z, Song H, Li P (2010) Potential antioxidant and anticoagulant capacity of low molecular weight fucoidan fractions extracted from Laminaria japonica. Int J Biol Macromol 46:6–12
Yaich H, Garna H, Besbes S, Barthélemy J-P, Paquot M, Blecker C, Attia H (2014) Impact of extraction procedures on the chemical, rheological and textural properties of ulvan from Ulva lactuca of Tunisia coast. Food Hydrocolloids 40:53–63
Yaich H, Ben AA, Abbes F, Bouaziz M, Besbes S, Richel A, Blecker C, Attia H, Garna H (2017) Effect of extraction procedures on structural, thermal and antioxidant properties of ulvan from Ulva lactuca collected in Monastir coast. Int J Biol Macromol 105:1430–1439. https://doi.org/10.1016/j.ijbiomac.2017.07.141
Yamamoto M (1980) Physicochemical studies on sulfated polysaccharides extracted from seaweeds at various temperatures. Agric Biol Chem 44:589–593
Yang X, Li A, Li X, Sun L, Guo Y (2020) An overview of classifications, properties of food polysaccharides and their links to applications in improving food textures. Trends Food Sci Technol 102:1–15
Yokoyama MY, Guimarães O (1975) Determinação dos teores de Na, K, P e proteínas em algumas algas marinhas. Acta Biol Paranaense 4:19–24
Zaki MA, Ashour M, Heneash AMM, Mabrouk MM, Alprol AE, Khairy HM, Nour AM, Mansour AT, Hassanien HA, Gaber A, Elshobary ME (2021) Potential applications of Native Cyanobacterium Isolate (Arthrospira platensis NIOF17/003) for biodiesel production and utilization of its byproduct in Marine Rotifer (Brachionus plicatilis) production. Sustainability 13:1769. https://doi.org/10.3390/su13041769
Acknowledgements
Authors are thankful to NIOF for funding this work during the project: "Antimicrobial applications of sulfated polysaccharides extracted from marine algae". Deeply, the authors thanks Dr. Mohammed Ibrahim; researcher of marine chemistry, NIOF, for helping explain chemical analyses.
Funding
Open access funding provided by The Science, Technology & Innovation Funding Authority (STDF) in cooperation with The Egyptian Knowledge Bank (EKB).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors confirm that they have no conflicts of interest with respect to the work described in this manuscript.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Barakat, K.M., Ismail, M.M., Abou El Hassayeb, H.E. et al. Chemical characterization and biological activities of ulvan extracted from Ulva fasciata (Chlorophyta). Rend. Fis. Acc. Lincei 33, 829–841 (2022). https://doi.org/10.1007/s12210-022-01103-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12210-022-01103-7