Abstract
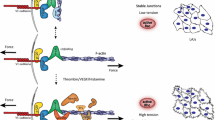
It has been well established that mechanical stimuli including fluid shear stress and cyclic stretch play a key role in endothelial cell (EC) remodeling. However, in contrast to global remodeling to these mechanical stimuli, little is known of how local mechanical forces are transmitted through cells to induce cell remodeling leading to alteration in cell functions. In this study, we demonstrated that EC remodeling can be exerted by local tension generated in a neighboring EC. In this technique, a glass microneedle was used to apply local stretch in an EC in confluent monolayer and the resulting tension is transmitted to a neighboring EC across intercellular junctions. Local stretch induced reorientation and elongation of ECs parallel to the direction of stretch associated with reorganization of stress fibers. In addition, recruitment of Src homology 2-containing tyrosine phosphatase-2, binding to intercellular adhesion molecules platelet-endothelial cellular adhesion molecules-1, was selectively observed at the force-transmitted intercellular junctions after application of local stretch. These findings suggest that intercellular junctions can not only transmit but also sense local forces, and are potentially involved in EC mechanotransduction pathways.







Similar content being viewed by others
References
Dewey CF, Bussplari SR, Gimbrone MA, Davies PF (1981) The dynamic response of vascular endothelial cells to fluid shear stress. J Biomech Eng 103:177–185.
Galbraith CG, Skalak R, Chien S (1998) Shear stress induces spatial reorganization of the endothelial cell cytoskeleton. Cell Motil Cytoskelet 40:317–330.
Sato M, Nagayama K, Kataoka N, Sasaki M, Hane K (2000) Local mechanical properties measured by atomic force microscopy for cultured bovine endothelial cells exposed to shear stress. J Biomech 33:127–135.
Kataoka N, Ujita S, Sato M (1998) The effect of flow direction on the morphological responses of cultured bovine aortic endothelial cells. Med Biol Eng Comput 36:122–128.
Shirinsky VP, Antonov AS, Birukov KG, Sobolevsky AV, Romanov YA, Kabaeva NV, Antonova GN, Smirnov VN (1989) Mechano-chemical control of human endothelium orientation and size. J Cell Biol 109:331–339.
Wang JH, Goldschmidt-Clermont P, Wille J, Yin FC (2001) Specificity of endothelial cell reorientation in response to cyclic mechanical stretching. J Biomech 34:1563–1572.
Sugaya Y, Sakamoto N, Ohashi T, Sato M (2003) Elongation and random orientation of bovine endothelial cells in response to hydrostatic pressure: comparison with response to shear stress. JSME Int J Ser C 46:1248–1255.
Ohashi T, Sugaya Y, Sakamoto N, Sato M (2007) Hydrostatic pressure influences morphology and expression of VE-cadherin of vascular endothelial cells. J Biomech 40:2399–2405.
Albelda SM, Muller WA, Buck CA, Newman PJ (1991) Molecular and cellular properties of PECAM-1 (endoCAM/CD31): a novel vascular cell-cell adhesion molecule. J Cell Biol 114:1059–1068.
Ilan N, Cheung L, Pinter E, Madri JA (2000) Platelet-endothelial cell adhesion molecule-1 (CD31), a scaffolding molecule for selected catenin family members whose binding is mediated by different tyrosine and serine/threonine phosphorylation. J Biol Chem 275:21435–21443.
Kataoka N, Ujita S, Kimura K, Sato M (1998) The morphological responses of cultured bovine aortic endothelial cells to fluid-imposed shear stress sparse and colony conditions. JSME Int J Ser C 41:76–82.
Masuda M, Fujiwara K (1993) Morphological responses of single endothelial cells exposed to physiological levels of fluid shear stress. Front Med Biol Eng 5:79–87.
Osawa M, Masuda M, Kusano K, Fujiwara K (2002) Evidence for a role of platelet endothelial cell adhesion molecule-1 in endothelial cell mechanosignal transduction: is it a mechanoresponsive molecule? J Cell Biol 158:773–785.
Tzima E, Irani-Tehrani M, Kiosses WB, Dejana E, Schultz DA, Engelhardt B, Cao G, DeLisser H, Schwartz MA (2005) A mechanosensary complex that mediates the endothelial cell response to fluid shear stress. Nature 437:426–431.
Sakamoto N, Ohashi T, Sato M (2001) Effect of magnetic field on nitric oxide synthesis of cultured endothelial cells. Int J Appl Electromagn Mech 14:317–322.
Sokabe M, Hayakawa K, Tatsumi H (2005) Varieties of mechanotransduction: the cytoskeletal stress fibre as a force transmitter and a mechanosensor. Proc Aust Physiol Soc 36:95.
Sasamoto A, Nagino M, Kobayashi S, Naruse K, Nimura Y, Sokabe M (2005) Mechanotransduction by integrin is essential for IL-6 secretion from endothelial cells in response to uniaxial continuous stretch. Am J Physiol, Cell Physiol 288:C1012–1022.
Ohashi T, Sugawara H, Matsumoto T, Sato M (2000) Surface topography measurement and intracellular stress analysis of cultured endothelial cells exposed to fluid shear stress. JSME Int J Ser C 43:780–786.
Tzima E, Del Pozo MA, Kiosses WB, Mohamed SA, Li S, Chien S, Schwartz MA (2002) Activation of Rac1 by shear stress in endothelial cells mediates both cytoskeletal reorganization and effects on gene expression. EMBO J 21:6791–6800.
Noria S, Xu F, McCue S, Jones M, Gotlieb AI, Langille BL (2004) Assembly and reorientation of stress fibers drives morphological changes to endothelial cells exposed to shear stress. Am J Pathol 164:1211–1223.
Li S, Chen BP, Azuma N, Hu YL, Wu SZ, Sumpio BE, Shyy JY, Chien S (1999) Distinct roles for the small GTPases Cdc42 and Rho in endothelial responses to shear stress. J Clin Invest 103:1141–1150.
Naruse K, Yamada T, Sokabe M (1998) Involvement of SA channels in orienting response of cultured endothelial cells to cyclic stretch. Am J Physiol 274:H1532–1538.
Yoshigi M, Clark EB, Yost HJ (2003) Quantification of stretch-induced cytoskeletal remodeling in vascular endothelial cells by image processing. Cytometry A 55:109–118.
Kaunas R, Nguyen P, Usami S, Chien S (2005) Cooperative effects of Rho and mechanical stretch on stress fiber organization. Proc Natl Acad Sci 102:15895–15900.
Acknowledgement
The authors thank Dr. Ikuo Takahashi for kindly providing human umbilical cords. This work was in part supported financially by Grants-in-Aid for Scientific Research from the Ministry of Education, Culture, Sports, Science and Technology (MEXT) in Japan (Nos. 15086203, 17200030, 17680036).
Author information
Authors and Affiliations
Corresponding author
Appendix. Calculation of SF parameters with image processing
Appendix. Calculation of SF parameters with image processing
The protocol for image processing to determine SF parameters is shown in Fig. 8. Algorithm based on pixel intensity of fluorescence images of GFP-actin was adapted from previous reports [23, 24]. SFs were extracted from original image [Fig. 8(a)] with convolution filter application, as shown in Fig. 8(b). For giving pixels p i ,j in 8-bit gray-scale, the pixels surrounding p i ,j were selected as a sample matrix [equation (1)]
Convolution using horizontal and vertical kernels [equations (2) and (3)], which define weighted sum of neighboring pixels, provided magnitude of brightness gradient of SFs in each pixel [equation (4)].
The pixel whose G value was bigger than 5-fold of averaged pixel intensity in whole area of the target cell was assigned as a constituent of SFs. In order to calculate the angle of SF orientation, the angle of minimal gradient in pixel intensity in each pixel [Fig. 8(c)] was calculated using Sobel kernels as previously reported [20]. The angle data allocated to only pixels which have been assigned as a constituent of SFs [Fig. 8(d)]. Angle values were summed up across the cell with vectorial summation and were assumed that each pixel has a vector consisted of the calculated angle and unit length. The angle between the direction of the resultant vector and the tensile direction was defined as the angle of SF orientation. The length of the resultant vector divided by the number of pixels was defined as the uniformity index of SFs, which indicate the degree of alignment of SFs. All calculation processes were executed on Excel 2004 for Mac (Microsoft, USA). Analyses were performed on images obtained at every 5 min after application of local stretch.
Rights and permissions
About this article
Cite this article
Ueki, Y., Sakamoto, N., Ohashi, T. et al. Morphological Responses of Vascular Endothelial Cells Induced by Local Stretch Transmitted Through Intercellular Junctions. Exp Mech 49, 125–134 (2009). https://doi.org/10.1007/s11340-008-9143-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11340-008-9143-3