Abstract
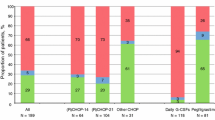
The long-term effects of pegfilgrastim administered in the first cycle of chemotherapy in day-to-day practice remain unclear. We retrospectively identified 114 patients aged ≥ 70 years with diffuse large B-cell lymphoma who received a rituximab-cyclophosphamide-doxorubicin-vincristine-prednisolone (R-CHOP) regimen in our institution. Twenty-six patients received pegfilgrastim (pegfilgrastim group); of the 88 patients scheduled to receive conventional granulocyte-colony stimulating factor (G-CSF) when their neutrophil count decreased (neut-adjusted-G group), conventional G-CSF was ultimately administered to 57. During the first cycle of R-CHOP, the incidence of febrile neutropenia was lower in the pegfilgrastim group than in the neut-adjusted-G group (0% vs. 18%, p = 0.020). Throughout all cycles, a higher proportion of patients exhibited sustained relative dose intensity (≥ 80%) in the pegfilgrastim group than in the neut-adjusted-G group (25% vs. 4.0%, p = 0.008). A lower proportion of patients received a reduced dose in the second cycle in the pegfilgrastim group than in the neut-adjusted-G group (0% vs. 10%, p = 0.116). Although the differences were not significant, the pegfilgrastim group showed higher progression-free survival and overall survival than the neut-adjusted-G group. Adequate prevention of febrile neutropenia using pegfilgrastim during the first cycle of R-CHOP may contribute to avoidance of dose intensity reduction in all cycles.


Similar content being viewed by others
References
Liu Y, Barta SK. Diffuse large B-cell lymphoma: 2019 update on diagnosis, risk stratification, and treatment. Am J Hematol. 2019;94(5):604–16.
Westin EH, Longo DL. Lymphoma and myeloma in older patients. Semin Oncol. 2004;31(2):198–205.
Coiffier B, Lepage E, Briere J, Herbrecht R, Tilly H, Bouabdallah R, et al. CHOP chemotherapy plus rituximab compared with CHOP alone in elderly patients with diffuse large-B-cell lymphoma. N Engl J Med. 2002;346(4):235–42.
Lyman GH, Poniewierski MS, Culakova E. Risk of chemotherapy-induced neutropenic complications when treating patients with non-Hodgkin lymphomas. Expert Opin Drug Saf. 2016;15(4):483–92.
Yamaguchi H, Hirakawa T, Inokuchi K. Importance of relative dose intensity in chemotherapy for diffuse large B-cell lymphoma. J Clin Exp Hematopathol. 2011;51(1):1–5.
Yamamoto M, Suzuki I, Saitou K, Tsumanuma R, Okuyama S, Kumagai H, et al. Impact of comorbidity and relative dose intensity on outcomes in diffuse large B-cell lymphoma patients treated with R-CHOP. J Cancer Res Clin Oncol. 2020;146(11):2995–3002.
Wildiers H, Reiser M. Relative dose intensity of chemotherapy and its impact on outcomes in patients with early breast cancer on aggressive lymphoma. Crit Rev Oncol Hematol. 2011;77(3):221–40.
Holmes FA, Jones SE, O’Shaughnessy J, Vukelja S, George T, Savin M, et al. Comparable efficacy and safety profiles of once-per-cycle pegfilgrastim and daily injection filgrastim in chemotherapy-induced neutropenia: a multicenter dose-finding study in women with breast cancer. Ann Oncol. 2002;13(6):903–9.
Tai E, Guy GP, Dunbar A, Richardson LC. Cost of cancer-related neutropenia or fever hospitalizations, United States, 2012. J Oncol Pract. 2017;13(6):e552-561.
Mehta HM, Malandra M, Corey SJ. G-CSF and GM-CSF in Neutropenia. J Immunol. 2015;195(4):1341–9.
Crawford J, Becker PS, Armitage JO, Blayney DW, Chavez J, Curtin P, et al. Myeloid growth factors, Version 2.2017, NCCN clinical practice guidelines in oncology. J Natl Compr Cancer Netw. 2017;15(12):1520–41.
Yokoyama M, Kusano Y, Nishihara A, Inoue N, Nishimura N, Mishima Y, et al. Incidence and risk factors for febrile neutropenia in Japanese patients with non-Hodgkin B cell lymphoma receiving R-CHOP: 2-year experience in a single center (STOP FN in NHL 2). Support Care Cancer. 2020;28(2):571–9.
Almenar D, Mayans J, Juan O, Bueno JM, Lopez JI, Frau A, et al. Pegfilgrastim and daily granulocyte colony-stimulating factor: patterns of use and neutropenia-related outcomes in cancer patients in Spain-results of the learn study. Eur J cancer Care. 2009;18(3):280–6.
Matsuda K, Taisuke J, Miyauchi M, Toyama K, Nakazaki K, Matsui H, et al. Primary prophylaxis with pegfilgrastim in patients with newly-diagnosed diffuse large B-cell lymphoma: propensity score and instrumental variable analyses. Leuk Lymphoma. 2020;61(10):2435–41.
Hirakawa T, Yamaguchi H, Yokose N, Gomi S, Inokuchi K, Dan K. Importance of maintaining the relative dose intensity of CHOP-like regimens combined with rituximab in patients with diffuse large B-cell lymphoma. Ann Hematol. 2010;89(9):897–904.
Lyman GH, Dale DC, Crawford J. Incidence and predictors of low dose-intensity in adjuvant breast cancer chemotherapy: a nationwide study of community practices. J Clin Oncol. 2003;21(24):4524–31.
Crawford J, Dale DC, Kuderer NM, Culakova E, Poniewierski MS, Wolff D, et al. Risk and timing of neutropenic events in adult cancer patients receiving chemotherapy: the results of a prospective nationwide study of oncology practice. J Natl Compr Canc Netw. 2008;6(2):109–18.
Vogel CL, Wojtukiewicz MZ, Carroll RR, Tjulandin SA, Barajas-Figueroa LJ, Wiens BL, et al. First and subsequent cycle use of pegfilgrastim prevents febrile neutropenia in patients with breast cancer: a multicenter, double-blind, placebo-controlled phase III study. J Clin Oncol. 2005;23(6):1178–84.
Culakova E, Thota R, Poniewierski MS, Kuderer NM, Wogu AF, Dale DC, et al. Patterns of chemotherapy-associated toxicity and supportive care in US oncology practice: a nationwide prospective cohort study. Cancer Med. 2014;3(2):434–44.
Chan A, Lee CP, Chiang J, Ng R. Breakthrough febrile neutropenia and associated complications among elderly cancer patients receiving myelosuppressive chemotherapy for solid tumors and lymphomas. Support Care Cancer. 2013;21(8):2137–43.
Mosteller RD. Simplified calculation of body-surface area. N Engl J Med. 1987;317(17):1098.
Kanda Y. Investigation of the freely available easy-to-use software ‘EZR’ for medical statistics. Bone Marrow Transplant. 2013;48(3):452–8.
Lyman GH, Morrison VA, Dale DC, Crawford J, Delgado DJ, Fridman M, et al. Risk of febrile neutropenia among patients with intermediate-grade non-Hodgkin’s lymphoma receiving CHOP chemotherapy. Leuk Lymphoma. 2003;44(12):2069–76.
Vose JM, Crump M, Lazarus H, Emmanouilides C, Schenkein D, Moore J, et al. Randomized, multicenter, open-label study of pegfilgrastim compared with daily filgrastim after chemotherapy for lymphoma. J Clin Oncol. 2003;21(3):514–9.
Grigg A, Solal-Celigny P, Hoskin P, Taylor K, McMillan A, Forstpointner R, et al. Open-label, randomized study of pegfilgrastim vs daily filgrastim as an adjunct to chemotherapy in elderly patients with non-Hodgkin’s lymphoma. Leuk Lymphoma. 2003;44(9):1503–8.
Kubo K, Miyazaki Y, Murayama T, Shimazaki R, Usui N, Urabe A, et al. A randomized, double blind trial of pegfilgrastim versus filgrastim for the management of neutropenia during CHASE(R) chemotherapy for malignant lymphoma. Br J Haematol. 2016;174(4):563–70.
Smith TJ, Bohlke K, Lyman GH, Carson KR, Crawford J, Cross SJ, et al. Recommendations for the use of WBC growth factors: American Society of Clinical Oncology clinical practice guideline update. J Clin Oncol. 2015;33(28):3199–212.
Acknowledgments
The authors would like to thank medical affairs division of the University of Tokyo hospital for calculating medical costs, Editage (www.editage.com) for English language editing.
Author information
Authors and Affiliations
Contributions
MI and KM designed the research. KM, AS, YM and MK advised the research design and analyses. MI collected the patient’s data and analyzed the data. MI and KM wrote the paper. All authors revised the manuscript and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
Kensuke Matsuda received the lecture fee from Kyowa Kirin. Arika Shimura received the lecture fee from Eisai. Yosuke Masamoto received the lecture fee from Janssen Pharmaceutical, Otsuka Pharmaceutical, Nihon Pharmaceutical, Nippon Shinyaku, Eisai, Bristol-Myers Squibb, Ono Pharmaceutical, Celgene, and Kyowa Kirin. Mineo Kurokawa received the lecture fee from MSD, Astellas, AbbVie, Amgen, Sanwa Chemistry, Shire Japan, Daiichi Sankyo, Sumitomo Dainippon Pharma, Takeda Pharmaceutical, Chugai Pharmaceutical, Boehringer Ingelheim, Janssen Pharmaceutical, Otsuka Pharmaceutical, Nippon Shinyaku, Eisai, Bioverativ Japan, Ono Pharmaceutical, Celgene, and Kyowa Kirin. Mineo Kurokawa received the research funding from Pfizer, Otsuka Pharmaceutical, Chugai Pharmaceutical, Astellas, Kyowa Kirin, Takeda Pharmaceutical, MSD, Teijin, Eisai, Sumitomo Dainippon Pharma, Nippon Shinyaku, Daiichi Sankyo, Ono Pharmaceutical, and AbbVie. None of these is related to the current study.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
About this article
Cite this article
Ise, M., Matsuda, K., Shimura, A. et al. Primary prophylaxis with pegfilgrastim during the first cycle of R-CHOP to avoid reduction of dose intensity in elderly patients. Int J Hematol 113, 823–831 (2021). https://doi.org/10.1007/s12185-021-03118-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12185-021-03118-6