Abstract
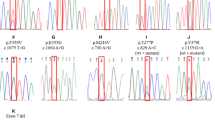
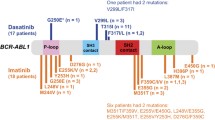
Kinase domain (KD) mutations of ABL1 represent the most common resistance mechanism to tyrosine kinase inhibitors (TKI) in CML. Besides T315I, mutations in codon 255 are highly resistant mutations in vitro to all TKI. We aimed to study the incidence, prognosis, and response to treatment in patients with E255K/V. We evaluated 976 patients by sequencing of BCR–ABL1 fusion transcript for ABL1 KD mutations. We identified KD mutations in 381 (39%) patients, including E255K/V in 48 (13% of all mutations). At mutation detection, 14 patients (29%) were in chronic phase (CP), 12 (25%) in accelerated phase (AP), and 22 (46%) in blast phase (BP). 9/14 CP patients responded to treatment (best response complete hematologic response—CHR-4; complete cytogenetic response—CCyR-1; major molecular response—MMR-4); only 4/12 AP patients (CHR 3; MMR 1) and 7/22 BP patients responded (CCyR 2; MMR 2; partial cytogenetic response—PCyR-3). After a median follow-up of 65 months from mutation detection, 36 patients (75%) died: 9/14 (64%) in CP, 9/12 (75%) in AP, and 18/22 (82%) in BP (p = 0.003); median overall survival was 12 months. Patients with E255K/V mutation have a poor prognosis, regardless of the stage of the disease at detection.


Similar content being viewed by others
References
Cortes J, Jabbour E, Kantarjian H, Yin CC, Shan J, O’Brien S, et al. Dynamics of BCR–ABL kinase domain mutations in chronic myeloid leukemia after sequential treatment with multiple tyrosine kinase inhibitors. Blood. 2007;110:4005–11.
Branford S, Rudzki Z, Walsh S, Parkinson I, Grigg A, Szer J, et al. Detection of BCR–ABL mutations in patients with CML treated with imatinib is virtually always accompanied by clinical resistance, and mutations in the ATP phosphate-binding loop (P-loop) are associated with a poor prognosis. Blood. 2003;102:276–83.
Soverini S, Martinelli G, Rosti G, Bassi S, Amabile M, Poerio A, et al. ABL mutations in late chronic phase chronic myeloid leukemia patients with up-front cytogenetic resistance to imatinib are associated with a greater likelihood of progression to blast crisis and shorter survival: a study by the GIMEMA Working Party on Chronic Myeloid Leukemia. J Clin Oncol. 2005;23:4100–9.
Jabbour E, Kantarjian H, Jones D, Talpaz M, Bekele N, O’Brien S, et al. Frequency and clinical significance of BCR–ABL mutations in patients with chronic myeloid leukemia treated with imatinib mesylate. Leukemia. 2006;20:1767–73.
Donato NJ, Wu JY, Stapley J, Lin H, Arlinghaus R, Aggarwal BB, et al. Imatinib mesylate resistance through BCR–ABL independence in chronic myelogenous leukemia. Cancer Res. 2004;64:672–7.
Gorre ME, Mohammad M, Ellwood K, Hsu N, Paquette R, Rao PN, et al. Clinical resistance to STI-571 cancer therapy caused by BCR–ABL gene mutation or amplification. Science. 2001;293:876–80.
Jabbour E, Jones D, Kantarjian HM, O’Brien S, Tam C, Koller C, et al. Long-term outcome of patients with chronic myeloid leukemia treated with second-generation tyrosine kinase inhibitors after imatinib failure is predicted by the in vitro sensitivity of BCR–ABL kinase domain mutations. Blood. 2009;114:2037–43.
Burgess MR, Skaggs BJ, Shah NP, Lee FY, Sawyers CL. Comparative analysis of two clinically active BCR–ABL kinase inhibitors reveals the role of conformation-specific binding in resistance. Proc Natl Acad Sci USA. 2005;102:3395–400.
von Bubnoff N, Manley PW, Mestan J, Sanger J, Peschel C, Duyster J. Bcr–Abl resistance screening predicts a limited spectrum of point mutations to be associated with clinical resistance to the Abl kinase inhibitor nilotinib (AMN107). Blood. 2006;108:1328–33.
Bradeen HA, Eide CA, O’Hare T, Johnson KJ, Willis SG, Lee FY, et al. Comparison of imatinib mesylate, dasatinib (BMS-354825), and nilotinib (AMN107) in an N-ethyl-N-nitrosourea (ENU)-based mutagenesis screen: high efficacy of drug combinations. Blood. 2006;108:2332–8.
Nicolini FE, Corm S, Le QH, Sorel N, Hayette S, Bories D, et al. Mutation status and clinical outcome of 89 imatinib mesylate-resistant chronic myelogenous leukemia patients: a retrospective analysis from the French intergroup of CML (Fi(phi)-LMC GROUP). Leukemia. 2006;20:1061–6.
Khorashad JS, de Lavallade H, Apperley JF, Milojkovic D, Reid AG, Bua M, et al. Finding of kinase domain mutations in patients with chronic phase chronic myeloid leukemia responding to imatinib may identify those at high risk of disease progression. J Clin Oncol. 2008;26:4806–13.
Griswold IJ, MacPartlin M, Bumm T, Goss VL, O’Hare T, Lee KA, et al. Kinase domain mutants of Bcr–Abl exhibit altered transformation potency, kinase activity, and substrate utilization, irrespective of sensitivity to imatinib. Mol Cell Biol. 2006;26:6082–93.
O’Hare T, Eide CA, Deininger MW. Bcr–Abl kinase domain mutations, drug resistance, and the road to a cure for chronic myeloid leukemia. Blood. 2007;110:2242–9.
O’Hare T, Shakespeare WC, Zhu X, Eide CA, Rivera VM, Wang F, et al. AP24534, a pan-BCR–ABL inhibitor for chronic myeloid leukemia, potently inhibits the T315I mutant and overcomes mutation-based resistance. Cancer Cell. 2009;16:401–12.
Chan WW, Wise SC, Kaufman MD, Ahn YM, Ensinger CL, Haack T, et al. Conformational control inhibition of the BCR–ABL1 tyrosine kinase, including the gatekeeper T315I mutant, by the switch-control inhibitor DCC-2036. Cancer Cell. 2011;19:556–68.
Baccarani M, Saglio G, Goldman J, Hochhaus A, Simonsson B, Appelbaum F, et al. Evolving concepts in the management of chronic myeloid leukemia: recommendations from an expert panel on behalf of the European LeukemiaNet. Blood. 2006;108:1809–20.
Kantarjian H, Sawyers C, Hochhaus A, Guilhot F, Schiffer C, Gambacorti-Passerini C, et al. Hematologic and cytogenetic responses to imatinib mesylate in chronic myelogenous leukemia. N Engl J Med. 2002;346:645–52.
Cortes J, Talpaz M, O’Brien S, Jones D, Luthra R, Shan J, et al. Molecular responses in patients with chronic myelogenous leukemia in chronic phase treated with imatinib mesylate. Clin Cancer Res. 2005;11:3425–32.
Kaplan EL, Meier P. Non-parametric estimation from incomplete observations. J Am Stat Assoc. 1965;53:457–81.
Jabbour E, Kantarjian H, Jones D, Breeden M, Garcia-Manero G, O’Brien S, et al. Characteristics and outcomes of patients with chronic myeloid leukemia and T315I mutation following failure of imatinib mesylate therapy. Blood. 2008;112:53–5.
Jabbour E, Kantarjian HM, Jones D, Reddy N, O’Brien S, Garcia-Manero G, et al. Characteristics and outcome of chronic myeloid leukemia patients with F317L BCR–ABL kinase domain mutation after therapy with tyrosine kinase inhibitors. Blood. 2008;112:4839–42.
Redaelli S, Mologni L, Rostagno R, Piazza R, Magistroni V, Ceccon M, et al. Three novel patient-derived BCR/ABL mutants show different sensitivity to second and third generation tyrosine kinase inhibitors. Am J Hematol. 2012;87:E125–8.
Acknowledgements
Dr. Cortes has received research support from Ariad, BMS, Novartis, Pfizer, and Teva. Dr. Cortes is consultant for Ariad, Novartis, BMS, and Pfizer.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
About this article
Cite this article
Naqvi, K., Cortes, J.E., Luthra, R. et al. Characteristics and outcome of chronic myeloid leukemia patients with E255K/V BCR–ABL kinase domain mutations. Int J Hematol 107, 689–695 (2018). https://doi.org/10.1007/s12185-018-2422-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12185-018-2422-6