Abstract
Hydraulic fracturing technology can significantly increase oil production from tight oil formations, but performance data show that production declines rapidly. In the long term, it is necessary to increase the development efficiency of block matrix, surfactant-aided imbibition is a potential way. The current work aimed to explain comprehensively how surfactants can enhance the imbibition rate. Laboratory experiments were performed to investigate the effects of wettability, interfacial tension (IFT), and relative permeability as the key parameters underlying surfactant solution imbibition. Two different types of surfactants, sodium dodecyl sulfate and polyethylene glycol octylphenol ether, at varied concentrations were tested on reservoir rocks. Experimental results showed that the oil recovery rate increased with increased wettability alteration and IFT and decreased residual oil saturation. A mechanistic simulator developed in previous studies was used to perform parametric analysis after successful laboratory-scale validation. Results were proven by parametric studies. This study, which examined the mechanism and factors influencing surfactant solution imbibition, can improve understanding of surfactant-aided imbibition and surfactant screening.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
In recent years, the increasing demand for oil and gas has driven oil industry to pursue increased oil production from low-permeability reservoirs (e.g., tight oil reservoirs). Unconventional reservoirs are thus expected to play an increasingly important role in addressing energy demand (Song et al. 2015; Zhang et al. 2016). With the assistance of hydraulic fracturing and horizontal well completion technology, tight oil has been exploited commercially (Ding et al. 2014; Keshavarz et al. 2016; Yuan et al. 2015; Zhang et al. 2015). However, oil production data have shown that oil production from tight oil reservoirs can be improved initially due to hydraulic fracturing technology, but then declines rapidly after a few years (Behmanesh et al. 2015; Xu et al. 2015). Low recovery and high production decline rate resulted in a large amount of tight oil remaining in the matrix (Wang et al. 2018). Consequently, the enhancement of the development efficiency of matrix blocks is a key point and spontaneous imbibition is an important mechanism to enhance oil recovery in matrix blocks.
In order to investigate spontaneous imbibition in matrix blocks, a large number of scholars have carried out experiments and theoretical studies (Babadagli 2000, 2001; Bourbiaux and Kalaydjian 1990; Kantzas et al. 1997; Mattax and Kyte 1962). Spontaneous imbibition is a complex process which depends on many variables, including matrix shape factor, porosity, permeability, wettability, gravity, and oil viscosity (Babadagli 2002; Civan and Rasmussen 2003, 2005; Di Donato et al. 2006; Fernø et al. 2011; Hatiboglu and Babadagli 2007; Rangel-German and Kovscek 2002, 2003; Tavassoli et al. 2005; Yildiz et al. 2006). Considering these factors and to accurately predict the imbibition behavior in experiments or the field, scaling imbibition models have been proposed (Mattax and Kyte 1962; Meng et al. 2016).
Unfortunately, for tight oil rocks the spontaneous imbibition recovery without the presence of surfactant is very low. In order to obtain higher recovery, it is necessary to carry out surfactant-aided imbibition experiments. In recent years, numerous imbibition studies have been done on wettability alteration by surfactants. Sharma et al. (2011) used three types of surfactants to change the wettability of carbonate rocks from mixed wet to water wet. Their experimental results revealed that some of the dual-surfactant systems could effectively alter the carbonate rock wettability alteration and recover 70%–80% original oil in place (OOIP) during spontaneous imbibition, which is significantly higher than the results of tests without surfactant (29%–40%). In addition, the reduction in the interfacial tension (IFT) between oil and injection water (Fini et al. 2012) and the formation of emulsion (Jamaloei et al. 2010; Zargartalebi et al. 2015) plays an important role in enhancing oil recovery. However, the relations between IFT and spontaneous imbibition recovery are still in dispute. Adibhatla and Mohanty (2008) observed that surfactants which alter the rock wettability to a water-wet regime give higher recovery rates for higher IFT systems, while surfactants which cannot alter rock wettability give higher recovery for lower IFT systems. The IFT, however, is a driving force of spontaneous imbibition. Kathel and Mohanty (2013) and Wang et al. (2017) insist that higher interfacial tension is beneficial to spontaneous imbibition. Meanwhile, after adding surfactants, it is known that the relative permeability will change, but previous studies did not discuss the effect of the changing of relative permeability on spontaneous imbibition. Therefore, the imbibition mechanism of surfactant solutions is still worthy of further investigation.
In this study, laboratory experiments are conducted to study the effects of changing in wettability, IFT, and relative permeability on spontaneous imbibition after adding surfactants. Furthermore, numerical simulations are performed using an in-house simulator developed in previous studies (El-Amin et al. 2013). Simulation results are compared with experimental data, and various parameters are analyzed for their effect on oil recovery during spontaneous imbibition. The research results in this study can be useful for understanding spontaneous imbibition. What is more, the conclusion can guide the surfactant choice in surfactant-aided imbibition schemes.
2 Methodology
2.1 Materials
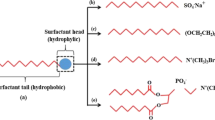
Sodium dodecyl sulfate (SDS, an anionic surfactant; Fig. 1a) and polyethylene (number of segments: 9.5) glycol octylphenol ether (Triton X-100, a nonionic surfactant; Fig. 1b) were used in this study. No cationic surfactants were used in this study due to the negative surface charge of sandstones. Surfactants were used as received, without any further purification.
Brine used was collected from the Xinjiang Oilfield, and its salinity was 35,120 mg/L. Table 1 presents a detailed composition of the formation water used. Cylindrical-shaped sandstone core samples, which were also obtained from the Xinjiang Oilfield, were 2.21–4.69 cm in length and 2.5 cm in diameter. Four core samples were cleaned with chemicals in sequence (toluene, benzene, trichloromethane, chloroform, acetone, xylene) and dried at 105 °C for 8 h before use, and their characteristics are listed in Table 2. The XRD results of Cores H-39-1 and H-56-1 are shown in Table 3. A mixture of kerosene and crude oil was used. The mixed oil had a viscosity of 2.3 cP at 25 °C and a specific gravity of 0.813. Surfactant solutions were prepared with formation water.
2.2 Contact angle measurement
To investigate the effect of surfactants on wettability alteration, the contact angles between oil and surfactant solutions of different surfactant concentrations (0.03 wt%, 0.05 wt%, and 0.07 wt%) were measured. Several methods have been developed to measure the contact angle, but the most commonly used method in the petroleum industry is the sessile drop method (Castillo 2011). In this method, the rock slice is placed horizontally in water, and a drop of oil is placed at the bottom of the rock slice. A photograph of the system is taken to accurately measure the contact angle θ (Tiab and Donaldson 2004). When θ is between 180° and 105°, the system is defined as oil wet. When θ is between 0° and 75°, the system is water wet. When θ is between 75° and 105°, the system is neutral wet (Anderson 1986; Elmofty 2012). Before each test, the rock slice was first aged in the mixed oil for about 7 days at 80 °C and then immersed in the surfactant solution for approximately 2 days. In this case, the role of different surfactants on wettability alteration would be quite different.
2.3 IFT measurement
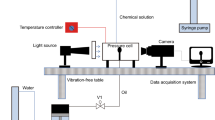
The IFT between the surfactant solution and the oil phase was measured with a IFT-820 instrument (TEMCO) (Fig. 2). The interfacial properties of fluids can be obtained from the shape of a pendant drop using the axisymmetric drop shape analysis (ADSA) technique. A glass cuvette was filled with the bulk phase of the surfactant solution and placed in an environmental chamber. IFT tests were conducted at room temperature. Oil drops were created in the surfactant bulk using a stainless steel needle connected to a small container filled with oil. As the glass cuvette was illuminated from the front with a lamp, the image can be captured by a video camera from the back of the chamber. The IFT values were then calculated through ADSA using an advanced drop shape analysis software based on the Young–Laplace equation.
2.4 Relative permeability measurement
Two types of measurement technique, steady-state displacement and unsteady-state displacement, have been used in previous studies to obtain the relative permeability curves. In this study, the unsteady-state displacement experiment was used to determine the relative permeability curve (GB/T 28912-2012). The primary experimental procedures were as follows:
-
(1)
Two cores were evacuated and then saturated with formation water.
-
(2)
The formation water was continuously injected into the core sample until the flow rate at the outlet end was stabilized. The pressure and the water volume were measured and recorded to calculate the relative water permeability. In this step, the pressure and water volume were measured three times to ensure that their relative errors are below 3%.
-
(3)
Mixed oil was injected to displace the formation water at an injection rate of 0.05 mL/min. The pressure and the water and oil volumes were recorded until no water could flow out from the core (or the flow rate at the outlet was stabilized).
-
(4)
The surfactant solution was then injected to displace the mixed oil at an injection rate of 0.05 mL/min. The pressure and the oil and water volumes were measured until no oil could flow out from the core (the flow rate at the outlet end was stabilized).
-
(5)
The core was cleaned with chemicals in sequence (toluene, benzene, trichloromethane, chloroform, acetone, xylene) and dried at 105 °C for 8 h. Change the concentration of surfactant solutions in Step 4. Repeat Steps (1)–(4). Draw the relative permeability curves at different surfactant concentrations (0, 0.03 wt%, and 0.05 wt%).
Figure 3 shows a schematic of the apparatus for measuring relative permeability, which was designed for two-phase (oil and water) relative permeability measurements. Cores H-39-1 and H-56-1 were used in water/oil relative permeability tests.
This experimental setup consisted of an Teledyne ISCO pump, two piston accumulators, a core holder, a confining pressure system, a liquid meter, and a computer system for data acquisition and process control. The core displacement system was placed in an oven to ensure that the experiment could be carried out at the desired temperature. The ISCO pump was used to drive the pistons in the transfer vessels that inject fluids into the core samples. A special pipeline (Fig. 4) was used to collect produced fluids. The smallest readable volume of liquids was 0.002 mL. By measuring the volume of oil and water displaced from the core sample, the production data would be recorded dynamically.
2.5 Spontaneous imbibition test
In spontaneous imbibition tests, the core samples were entirely soaked in the surfactant solutions of different concentrations at 25 °C (as shown in Fig. 5). The detailed experimental procedures are as follows:
-
(1)
Before tests, the core samples were cleaned with chemicals in sequence (toluene, benzene, trichloromethane, chloroform, acetone, xylene) and dried at 105 °C for 8 h, following the standard GB/T 29172-2012.
-
(2)
The sample was evacuated and then saturated with formation water.
-
(3)
The water-saturated core sample was placed in a core holder, and the mixed oil was injected into the core sample until the flow rate at the outlet end became stabilized. Initial oil saturation was calculated from the mass change of the core before and after displacement.
-
(4)
The core sample was then aged in the mixed oil at 80 °C for about 7 days.
-
(5)
The spontaneous imbibition test was conducted on the aged core sample. The core sample was weighted at different imbibition times.
-
(6)
By repeating the above-mentioned Steps (1)–(5), the change of core mass could be obtained versus soaking time. After the experiment, the figure of imbibition recovery versus time could be obtained. A total of 12 imbibition tests were conducted. The core properties and fluids used in different tests are listed in Table 4. In Test 11 and Test 12, the brine salinity was halved.
Table 4 Properties of the cores and fluids used in imbibition tests
3 Results and discussion
Several factors affect the imbibition recovery when surfactants are used. These influencing factors include temperature, pressure, salinity, relative permeability, and surface charges on the reservoir rock. In this study, temperature and pressure were kept constant. By means of the experiment and model, contact angle, IFT, and relative permeability were analyzed as the main parameters affecting the efficiency of imbibition.
3.1 Contact angle
Figure 6 shows pictures of oil droplets on the thin rock slice completely aged with the mixed oil and submerged in the surfactant solution. Table 5 presents the contact angle values. Evidently, both surfactants (SDS and Triton X-100) altered the rock wettability. In the anionic surfactant (SDS) solution, the wettability of the rock surface changed from strongly oil wet to water wet. However, the nonionic surfactant (Triton X-100) solution was unable to make the rock surface water wet but only to make the rock surface neutral wet. In addition, the higher the surfactant concentration, the smaller the contact angle was. The 0.05 wt% SDS solution was tested at different salinities (35,120 and 17,560 mg/L), and the contact angles observed were nearly the same at both salinities. Two different salinities were then thoroughly studied in spontaneous imbibition tests to analyze the effect of IFT.
3.2 IFT
Figure 7 shows the IFT between the aqueous and oleic phases as a function of the surfactant concentration in the mixed oil–surfactant solution systems. The experiments were conducted at atmospheric pressure (0.1 MPa) and 25 °C. The viscosity of the mixed oil used in the IFT test was 2.3 cP, while the salinity of formation water was 35,120 mg/L. For both surfactant solutions, IFT decreased with an increase in the surfactant concentration, which is due to further dissolution of the surfactant in water. As a result, more surfactant molecules tend to move toward the interface at higher surfactant concentrations, thus lowering the IFT. In addition, the IFT between the mixed oil and the 0.05 wt% surfactant solution (SDS) was tested at a low salinity of 17,560 mg/L, which was approximately 14.24 mN/m.
3.3 Relative permeability curves
Figures 8 and 9 show that the relative permeability to oil increased with the surfactant concentration, but the relative permeability to water remained almost constant for both the SDS solution and the Triton X-100 solution. Once the surfactant solution was used in the displacement tests, the residual oil saturation decreased significantly. The residual oil saturation decreased as the surfactant concentration increased. The relative permeability curve reflects the change of flow resistance in the spontaneous imbibition. The influence of relative permeability will be further studied in the following theoretical model simulation.
3.4 Spontaneous imbibition
Figure 10 shows that the oil recovery was a function of time for 12 spontaneous imbibition tests performed. Tests 1 and 2 were conducted with formation water, and it took 6 and 8 days before the core weight changed. The oil recovery was 7.6% and 9.6%, respectively, which suggests that the matrix is predominately oil wet.
Four imbibition tests (Tests 5, 6, 9, and 10) were performed with the Triton X-100 solution (nonionic surfactant). The oil recovery was higher than those of the cases with formation water. This suggests that the change of relative permeability and the decrease in the residual oil saturation can help imbibition (Fig. 9).
Six imbibition tests (Tests 3, 4, 7, 8, 11, and 12) were performed with SDS solutions (anionic surfactant). Among the six tests, the lowest final oil recovery was obtained at a low surfactant concentration of 0.03 wt% and a high salinity of 35,120 mg/L (Fig. 10c), while a significantly high oil recovery was obtained at a high surfactant concentration of 0.05 wt% and low salinity of 17,560 mg/L (Fig. 11). However, in the six cases with the anionic surfactant (SDS), the recovery was high (27.8%–36.1%), and it shows that wettability alteration plays an important role in the process of spontaneous imbibition. As in the conclusion obtained from Sect. 3.1, the anionic surfactant is effective as a wettability altering agent.
Regardless of the type of surfactant used, the final oil recovery by spontaneous imbibition increased gradually as the surfactant concentration increased. In addition, the difference in pore structures will also affect the oil recovery.
Figure 11 shows the oil recovery curves for spontaneous imbibition tests performed with the 0.05 wt% SDS solution of different salinities. As a result of the salinity difference, the IFT values between the aqueous and oleic phases were 7.51 and 14.24 mN/m, respectively, at salinities of 35,120, and 17,560 mg/L. The IFT increased as the brine salinity decreased at the same SDS concentration. The higher the IFT, the higher the oil recovery rate. In addition, the final oil recovery was higher for the higher case in both cases.
3.5 Simulation
In laboratory tests, it is difficult to use just a single variable in spontaneous imbibition tests. For example, when investigating the effect of contact angle in spontaneous imbibition, it is difficult to keep the IFT and residual oil saturation at a constant value. Therefore, imbibition tests cannot sufficiently explain the enhancement of the imbibition recovery mechanism of surfactants. In this section, a mechanistic simulator developed in previous studies (El-Amin et al. 2013) was used to perform parametric analysis after successful laboratory-scale validation. Spontaneous imbibition of brine into an oil-saturated core with all faces open for fluid exchange was modeled. Regardless of the gravity effect, the normalized oil saturation at any point (x, y, z) in the core sample at any time can be described by a hyperbolic partial differential equation. By the difference method, a numerical solution of this problem of two-phase immiscible flow in porous media was introduced.
where S is the normalized oil saturation, m3/m3; Sw is the normalized water saturation, m3/m3; Kro is the relative permeability to oil, fraction; Krw is the relative permeability to water, fraction; \(\mu_{\text{o}}\) is the oil viscosity, Pa s; \(\mu_{\text{w}}\) is the water viscosity, Pa s; pc is the capillary pressure, Pa; t is the imbibition time, s; and ϕ is the fractional porosity, m3/m3.
The influence of the surfactant, consequent IFT, contact angle, and relative permeability changes were considered in the numerical model.
The key input parameters include capillary pressure (pc) and relative permeability values (Kro, Krw) and their variation with the surfactant concentration. Capillary pressure is assumed to depend upon IFT, contact angle, and saturation through a power-law model.
In Eq. (3), a and b are empirical parameters that can be obtained from the mercury injection curve; σ is the IFT; and θ is the contact angle. For the same core, porosity (ϕ) and permeability (k) are constant. Relative permeability curves are described by a modified Brooks–Corey model as follows:
where Kroe corresponds to the wetting phase end-point relative permeability; Krwe corresponds to the non-wetting phase end-point relative permeability; no is the exponential parameter for the wetting phase; and nw is the exponential parameter for the non-wetting phase; Sor is the residual oil saturation, m3/m3; Swi is the irreducible water saturation, m3/m3; and Sw(i) is the water saturation at any point, m3/m3.
The MATLAB code “nlinfit” uses the Levenberg–Marquardt algorithm for nonlinear least squares to compute non-robust fits. “Permeability, porosity, Sor, Swi, θ, σ” were obtained directly from experimental data, while “Kroe, Krwe, no, nw, a, b” were obtained by fitting. Variations in the IFT, contact angle, and relative permeability with the surfactant concentration are modeled with all other parameters obtained from the experimental data. The values of different parameters used in the simulation are provided in Table 6.
The reliability of the simulator is established by comparing the core-scale simulation results to the laboratory-scale results from Test 8 (Fig. 11). Through simulations, we have analyzed the effect of three parameters, namely extent of wettability alteration, IFT, and residual oil saturation, on the amount and rate of oil recovery.
Figure 12 shows a comparison between numerical simulation results and those of Test 8. A good match was observed between the simulated oil recovery and the experimental data.
The effect of wettability alteration on oil recovery was studied by changing the contact angle from 85° to 55°. In the experiment, the other parameters (except for the wettability) were the same in all cases. Figure 13 shows that the oil recovery increases with increasing the extent of wettability alteration. The capillary pressure increases as the contact angle decreases. The capillary pressure is the only driving force in the spontaneous imbibition. Thus, the decrease in contact angle leads to a high oil recovery.
Figure 14 shows simulation results obtained by varying the IFT, from 5 to 20 mN/m. As the IFT increases, the capillary pressure and the oil recovery rate increase. The simulation results were consistent with the experimental results. “The higher IFT, the higher the rate of oil recovery” conclusion is obtained only if other parameters remain unchanged. In addition, two published articles have come to the same conclusion. Kathel and Mohanty (2013) explained the impact of IFT through experiments, and Wang et al. (2017) verified the same conclusion by numerical simulation. Thus, the selection of surfactant was expected to be able to maintain a certain IFT.
Figure 15 shows the effect of the change in the residual oil saturation on the oil recovery. A decrease in residual oil saturation leads to high oil recovery rates. As the residual oil saturation decreases, the relative permeability curve moved to the right (Figs. 8, 9), and the resistance to fluid flow in spontaneous imbibition tests decreases.
4 Conclusions
In order to investigate the performance of spontaneous imbibition enhanced by surfactants in tight oil reservoirs, spontaneous imbibition experiments with different concentrations of various surfactants were carried out, and the basic parameters (wettability, interfacial tension, relative permeability) were also tested. At the same time, a mechanistic simulator developed in previous studies was used to perform parametric analysis after successful laboratory-scale validation. Through the study, the following three conclusions are obtained.
-
(1)
The anionic and nonionic surfactants can alter rock wettability. However, the anionic surfactant (SDS) could alter the rock wettability from strongly oil wet to water wet, while the nonionic surfactant (Triton X-100) was unable to make the rock slices of water wet type but only caused a large decrease in oil wetness.
-
(2)
The oil relative permeability increased as the surfactant solution concentration increased, while the water relative permeability remained almost constant. A high oil recovery rate was observed during imbibition tests with surfactant in tight sandstones.
-
(3)
Parametric studies performed using numerical simulation show that the oil recovery rate increased with increased wettability alteration, increased IFT, and decreased residual oil saturation. This study comprehensively explains how surfactants can enhance imbibition rate and suggests that surfactants that can increase wettability alteration, decrease residual oil saturation, and maintain a high IFT enhance oil recovery.
References
Adibhatla B, Mohanty KK. Parametric analysis of surfactant-aided imbibition in fractured carbonates. J Colloid Interface Sci. 2008;317(2):513–22. https://doi.org/10.1016/j.jcis.2007.09.088UR.
Anderson WG. Wettability literature survey—part 2: wettability measurement. J Pet Technol. 1986;38(11):1246–62. https://doi.org/10.2118/13933-PA.
Babadagli T. Efficiency of capillary imbibition dominated displacement of non-wetting phase by wetting phase in fractured porous media. Transp Porous Media. 2000;40:314–44. https://doi.org/10.1023/A:1006777703905.
Babadagli T. Scaling of co-current and counter-current capillary imbibition for surfactant and polymer injection in naturally fractured reservoirs. SPE J. 2001;6(4):465–78. https://doi.org/10.2118/74702-PA.
Babadagli T. Dynamics of capillary imbibition when surfactant, polymer, and hot water are used as aqueous phase for oil recovery. J Colloid Interface Sci. 2002;156:203–13. https://doi.org/10.1006/jcis.2001.8015UR.
Behmanesh H, Hamdi H, Clarkson CR. Production data analysis of tight gas condensate reservoirs. J Nat Gas Sci Eng. 2015;22:22–34. https://doi.org/10.1016/j.jngse.2014.11.005UR.
Bourbiaux BJ, Kalaydjian FJ. Experimental study of cocurrent and countercurrent flows in natural porous media. SPE Reserv Eng. 1990;5(3):361–8. https://doi.org/10.2118/18283-PA.
Castillo SC. Water and surfactant flooding at different wettability conditions. M.Sc. thesis. University of Stavanger, Faculty of Science and Technology; 2011.
Civan F, Rasmussen ML. Modeling and validation of hindered–matrix–fracture transfer for naturally fractured petroleum reservoirs. In: Production and operations symposium, 23–26 March, Oklahoma City, Oklahoma; 2003. https://doi.org/10.2118/80918-MS.
Civan F, Rasmussen ML. Determination of parameters for matrix–fracture transfer functions from laboratory data. In: SPE production and operation symposia, 17–19 April, Oklahoma City, OK; 2005. https://doi.org/10.1016/S0920-4105(01)00188-7.
Di Donato G, Tavassoli Z, Blunt MJ. Analytical and numerical analysis of oil recovery by gravity drainage. J Pet Sci Eng. 2006;54(1):55–69. https://doi.org/10.1016/j.petrol.2006.08.002UR.
Ding DY, Wu YS, Jeannin L. Efficient simulation of hydraulic fractured wells in unconventional reservoirs. J Pet Sci Eng. 2014;122:631–42. https://doi.org/10.1016/j.petrol.2014.09.005UR.
El-Amin MF, Salama A, Sun SY. Numerical and dimensional investigation of two-phase countercurrent imbibition in porous media. J Comput Appl Math. 2013;242:285–96. https://doi.org/10.1016/j.cam.2012.09.035UR.
Elmofty O. Surfactant enhanced oil recovery by wettability alteration in sandstone reservoirs. M.Sc. thesis. Missouri University of Science and Technology, USA; 2012.
Fernø MA, Haugen A, Graue A. Wettability effects on the matrix–fracture fluid transfer in fractured carbonate rocks. J Pet Sci Eng. 2011;77(1):146–53. https://doi.org/10.1016/j.petrol.2011.02.015UR.
Fini MF, Riahi S, Bahramian A. Experimental and QSPR studies on the effect of ionic surfactants on n-decane–water interfacial tension. J Surfactants Deterg. 2012;15(4):477–84. https://doi.org/10.1007/s11743-012-1330-7.
Hatiboglu CU, Babadagli T. Oil recovery by counter-current spontaneous imbibition: effects of matrix shape factor, gravity, IFT, oil viscosity, wettability, and rock type. J Pet Sci Eng. 2007;59(1):106–22. https://doi.org/10.1016/j.petrol.2007.03.005UR.
Jamaloei BY, Asghari K, Kharrat R, Ahmadloo F. Pore-scale two-phase filtration in imbibition process through porous media at high- and low-interfacial tension flow conditions. J Pet Sci Eng. 2010;72(3):251–69. https://doi.org/10.1016/j.petrol.2010.03.026UR.
Kantzas A, Pow M, Allsopp K, Marenette D. Co-current and counter-current imbibition analysis for tight fractured carbonate gas reservoirs. In: Technical meeting/petroleum conference of the South Saskatchewan Section, October 19–22, Regina, Saskatchewan, Canada; 1997. https://doi.org/10.2118/97-181.
Kathel P, Mohanty KK. Wettability alteration in a tight oil reservoir. Energy Fuels. 2013;27(11):6460–8. https://doi.org/10.1021/ef4012752.
Keshavarz A, Badalyan A, Johnson R Jr, Bedrikovetsky P. Productivity enhancement by stimulation of natural fractures around a hydraulic fracture using micro-sized proppant placement. J Nat Gas Sci Eng. 2016;33:1010–24. https://doi.org/10.1016/j.jngse.2016.03.065UR.
Mattax CC, Kyte JR. Imbibition oil recovery from fractured, water drive reservoir. SPE J. 1962;2(2):177–84. https://doi.org/10.2118/187-PA.
Meng QB, Liu HQ, Wang J, et al. Effect of gravity on spontaneous imbibition from cores with two ends open in the frontal flow period. J Pet Sci Eng. 2016;141:16–23. https://doi.org/10.1016/j.petrol.2016.01.024.
Rangel-German E, Kovscek AR. Experimental and analytical study of multidimensional imbibition in fractured porous media. J Pet Sci Eng. 2002;36:45–60. https://doi.org/10.1016/S0920-4105(02)00250-4UR.
Rangel-German E, Kovscek A.R. Time dependent matrix–fracture shape factors for partially and completely immersed fractures. In: SPE annual technology conference and exhibition, 5–8 October, Denver, CO; 2003. https://doi.org/10.1016/j.petrol.2006.08.004.
Sharma G, Hughes B, Mohanty KK. Wettability alteration in high-temperature and high-salinity carbonate reservoirs. In: SPE annual technical conference and exhibition, 30 October–2 November, Denver, Colorado; 2011. https://doi.org/10.2118/147306-MS.
Song Y, Li Z, Jiang L. The concept and the accumulation characteristics of unconventional hydrocarbon resources. Pet Sci. 2015;12(4):563–72. https://doi.org/10.1007/s12182-015-0060-7.
Tavassoli Z, Zimmerman RW, Blunt MJ. Analysis of counter-current imbibition with gravity in weakly water-wet systems. J Pet Sci Eng. 2005;48(1):94–104. https://doi.org/10.1016/j.petrol.2005.04.003UR.
Tiab D, Donaldson EC. Petrophysics: theory and practice of measuring reservoir rock and fluid transport properties. 2nd ed. New York: Elsevier; 2004. pp. 313–414.
Wang J, Liu HQ, Xia J, Liu YT, Hong C, Meng QB. Mechanism simulation of oil displacement by imbibition in fractured reservoirs. Pet Explor Dev. 2017;44(5):805–14. https://doi.org/10.1016/S1876-3804(17)30091-5UR.
Wang XZ, Peng XL, Zhang SJ, Du ZW, Zeng FH. Characteristics of oil distributions in forced and spontaneous imbibition of tight oil reservoir. Fuel. 2018;224:280–8. https://doi.org/10.1016/j.fuel.2018.03.104.
Xu JC, Guo CH, Teng WC, Wei MZ, Jiang RZ. Production performance analysis of tight oil/gas reservoirs considering stimulated reservoir volume using elliptical flow. J Nat Gas Sci Eng. 2015;26:827–39. https://doi.org/10.1016/j.jngse.2015.06.057UR.
Yildiz HO, Gokmen M, Cesur Y. Effect of shape factor, characteristics length, and boundary conditions on spontaneous imbibition. J Pet Sci Eng. 2006;53:158–70. https://doi.org/10.1016/j.petrol.2006.06.002UR.
Yuan B, Wood DS, Yu WQ. Stimulation and hydraulic fracturing technology in natural gas reservoirs: theory and case studies (2012–2015). J Nat Gas Sci Eng. 2015;26:1414–21. https://doi.org/10.1016/j.jngse.2015.09.001UR.
Zargartalebi M, Kharrat R, Barati N. Enhancement of surfactant flooding performance by the use of silica nanoparticles. Fuel. 2015;143:21–7. https://doi.org/10.1016/j.fuel.2014.11.040UR.
Zhang SC, Lei X, Zhou YS, Xu GQ. Numerical simulation of hydraulic fracture propagation in tight oil reservoirs by volumetric fracturing. Pet Sci. 2015;12(4):674–82. https://doi.org/10.1007/s12182-015-0055-4.
Zhang XS, Wang HJ, Ma F, Sun XC, Zhang Y, Song ZH. Classification and characteristics of tight oil plays. Pet Sci. 2016;13(1):18–33. https://doi.org/10.1007/s12182-015-0075-0.
Acknowledgements
This study was supported by the Natural Science Foundation of China (Grant No. 51574257) and National 973 Project (No. 2015CB250900). The authors greatly appreciate the Xinjiang Oilfield for its encouragement and assistance throughout the project and preparation of this paper.
Author information
Authors and Affiliations
Corresponding author
Additional information
Edited by Yan-Hua Sun
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Meng, Z., Yang, SL., Cui, Y. et al. Enhancement of the imbibition recovery by surfactants in tight oil reservoirs. Pet. Sci. 15, 783–793 (2018). https://doi.org/10.1007/s12182-018-0253-y
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12182-018-0253-y