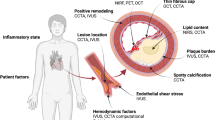
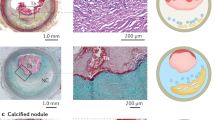
Abstract
Purpose of Review
Coronary plaque rupture is the dominant mechanism of acute myocardial infarction and sudden coronary death. If we are to make a major inroads on reducing morbidity and mortality from cardiovascular disease, more accurate prediction of an individual’s near-term cardiovascular risk (i.e., prediction of future coronary events) than what we can offer today is the ultimate goal to enable a more patient-tailored treatment strategy for coronary artery disease. In this review, we address the value of the vulnerable plaque concept in the context of an integrative cardiovascular risk assessment.
Recent Findings
Even with today’s state-of-the-art intravascular and non-invasive imaging, the identification of plaques at high-risk for rupture remains an elusive task albeit one in which important progress continues to be made. The vulnerable plaque concept emerged from the identification of morphological characteristics associated with plaque rupture as identified by autopsy studies. However, morphology alone may only be one of many factors which drive plaque progression. Therefore, the usefulness of the classical vulnerable plaque concept for predicting an individual’s cardiovascular risk clearly remains in the research realm. Nonetheless, the potential of being able to predict events before they happen remains an issue of utmost importance for cardiology.
Summary
The identification of patients at risk for adverse cardiovascular events requires a comprehensive risk assessment. Invasive and non-invasive coronary imaging techniques allow for a detailed detection of vulnerable plaque characteristics. In combination with systemic factors that increase the disease’s activity, the finding that an individual may harbor coronary plaques with vulnerable characteristics indicates elevated atherosclerotic disease risk and thus justifies a more intensive therapeutic approach.


Similar content being viewed by others
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Mozaffarian D, Benjamin EJ, Go AS, Arnett DK, Blaha MJ, Cushman M, et al. Executive summary: Heart Disease and Stroke Statistics--2016 update: a report from the American Heart Association. Circulation. 2016;133(4):447–54.
Bentzon JF, Otsuka F, Virmani R, Falk E. Mechanisms of plaque formation and rupture. Circ Res. 2014;114(12):1852–66.
Finn AV, Nakano M, Narula J, Kolodgie FD, Virmani R. Concept of vulnerable/unstable plaque. Arterioscler Thromb Vasc Biol. 2010;30(7):1282–92.
Virmani R, Kolodgie FD, Burke AP, Farb A, Schwartz SM. Lessons from sudden coronary death: a comprehensive morphological classification scheme for atherosclerotic lesions. Arterioscler Thromb Vasc Biol. 2000;20(5):1262–75.
Falk E, Nakano M, Bentzon JF, Finn AV, Virmani R. Update on acute coronary syndromes: the pathologists’ view. Eur Heart J. 2013;34(10):719–28.
Schaar JA, Muller JE, Falk E, Virmani R, Fuster V, Serruys PW, et al. Terminology for high-risk and vulnerable coronary artery plaques. Report of a meeting on the vulnerable plaque, June 17 and 18, 2003, Santorini, Greece. Eur Heart J. 2004;25(12):1077–82.
Otsuka F, Joner M, Prati F, Virmani R, Narula J. Clinical classification of plaque morphology in coronary disease. Nat Rev Cardiol. 2014;11(7):379–89.
Higuma T, Soeda T, Abe N, Yamada M, Yokoyama H, Shibutani S, et al. A combined optical coherence tomography and intravascular ultrasound study on plaque rupture, plaque erosion, and calcified nodule in patients with ST-segment elevation myocardial infarction: incidence, morphologic characteristics, and outcomes after percutaneous coronary intervention. JACC Cardiovasc Interv. 2015;8(9):1166–76.
Jia H, Abtahian F, Aguirre AD, Lee S, Chia S, Lowe H, et al. In vivo diagnosis of plaque erosion and calcified nodule in patients with acute coronary syndrome by intravascular optical coherence tomography. J Am Coll Cardiol. 2013;62(19):1748–58.
Farb A, Burke AP, Tang AL, Liang TY, Mannan P, Smialek J, et al. Coronary plaque erosion without rupture into a lipid core. A frequent cause of coronary thrombosis in sudden coronary death. Circulation. 1996;93(7):1354–63.
Burke AP, Farb A, Malcom GT, Liang Y, Smialek J, Virmani R. Effect of risk factors on the mechanism of acute thrombosis and sudden coronary death in women. Circulation. 1998;97(21):2110–6.
Virmani R, Burke AP, Farb A. Plaque rupture and plaque erosion. Thromb Haemost. 1999;82(Suppl 1):1–3.
Taylor AJ, Burke AP, O’Malley PG, Farb A, Malcom GT, Smialek J, et al. A comparison of the Framingham risk index, coronary artery calcification, and culprit plaque morphology in sudden cardiac death. Circulation. 2000;101(11):1243–8.
Campbell IC, Timmins LH, Giddens DP, Virmani R, Veneziani A, Rab ST, et al. Computational fluid dynamics simulations of hemodynamics in plaque erosion. Cardiovasc Eng Technol. 2013;4(4):464–73. https://doi.org/10.1007/s13239-013-0165-3.
• Kobayashi N, Takano M, Tsurumi M, Shibata Y, Nishigoori S, Uchiyama S, et al. Features and outcomes of patients with calcified nodules at culprit lesions of acute coronary syndrome: an optical coherence tomography study. Cardiology. 2018;139(2):90–100 This OCT study by Kobayashi et al. assessed culprit lesion plaque morphologies in ACS patients and classified them as calcified nodules, plaque rupture, and plaque erosion. Beneath the assessment of prevalences in a clinical setting, the main finding was that ACS patients with calcified nodules at the culprit lesion had more target lesion revascularization compared with those with plaque rupture or plaque erosion.
• Yahagi K, Kolodgie FD, Otsuka F, Finn AV, Davis HR, Joner M, et al. Pathophysiology of native coronary, vein graft, and in-stent atherosclerosis. Nat Rev Cardiol. 2016;13(2):79–98 Comprehensive review of the pathology of native coronary artery disease as well as that seen in vein grafts and in coronary stents.
Muller JE, Tofler GH, Stone PH. Circadian variation and triggers of onset of acute cardiovascular disease. Circulation. 1989;79(4):733–43.
Muller JE, Abela GS, Nesto RW, Tofler GH. Triggers, acute risk factors and vulnerable plaques: the lexicon of a new frontier. J Am Coll Cardiol. 1994;23(3):809–13.
Burke AP, Farb A, Malcom GT, Liang YH, Smialek J, Virmani R. Coronary risk factors and plaque morphology in men with coronary disease who died suddenly. N Engl J Med. 1997;336(18):1276–82.
Virmani R, Burke AP, Farb A, Kolodgie FD. Pathology of the vulnerable plaque. J Am Coll Cardiol. 2006;47(8 Suppl):C13–8.
Kolodgie FD, Burke AP, Farb A, Gold HK, Yuan J, Narula J, et al. The thin-cap fibroatheroma: a type of vulnerable plaque: the major precursor lesion to acute coronary syndromes. Curr Opin Cardiol. 2001;16(5):285–92.
Kolodgie FD, Virmani R, Burke AP, Farb A, Weber DK, Kutys R, et al. Pathologic assessment of the vulnerable human coronary plaque. Heart. 2004;90(12):1385–91.
Stary HC, Chandler AB, Dinsmore RE, Fuster V, Glagov S, Insull W Jr, et al. A definition of advanced types of atherosclerotic lesions and a histological classification of atherosclerosis. A report from the Committee on Vascular Lesions of the Council on Arteriosclerosis, American Heart Association. Circulation. 1995;92(5):1355–74.
Libby P, Schoenbeck U, Mach F, Selwyn AP, Ganz P. Current concepts in cardiovascular pathology: the role of LDL cholesterol in plaque rupture and stabilization. Am J Med. 1998;104(2a):14s–8s.
Burke AP, Virmani R, Galis Z, Haudenschild CC, Muller JE. 34th Bethesda Conference: Task force #2--What is the pathologic basis for new atherosclerosis imaging techniques? J Am Coll Cardiol. 2003;41(11):1874–86.
Cheruvu PK, Finn AV, Gardner C, Caplan J, Goldstein J, Stone GW, et al. Frequency and distribution of thin-cap fibroatheroma and ruptured plaques in human coronary arteries: a pathologic study. J Am Coll Cardiol. 2007;50(10):940–9.
Wang JC, Normand SL, Mauri L, Kuntz RE. Coronary artery spatial distribution of acute myocardial infarction occlusions. Circulation. 2004;110(3):278–84.
Falk E, Shah PK, Fuster V. Coronary plaque disruption. Circulation. 1995;92(3):657–71.
Shah PK, Falk E, Badimon JJ, Fernandez-Ortiz A, Mailhac A, Villareal-Levy G, et al. Human monocyte-derived macrophages induce collagen breakdown in fibrous caps of atherosclerotic plaques. Potential role of matrix-degrading metalloproteinases and implications for plaque rupture. Circulation. 1995;92(6):1565–9.
Moreno PR, Falk E, Palacios IF, Newell JB, Fuster V, Fallon JT. Macrophage infiltration in acute coronary syndromes. Implications for plaque rupture. Circulation. 1994;90(2):775–8.
Galis ZS, Sukhova GK, Lark MW, Libby P. Increased expression of matrix metalloproteinases and matrix degrading activity in vulnerable regions of human atherosclerotic plaques. J Clin Invest. 1994;94(6):2493–503.
Sukhova GK, Schonbeck U, Rabkin E, Schoen FJ, Poole AR, Billinghurst RC, et al. Evidence for increased collagenolysis by interstitial collagenases-1 and -3 in vulnerable human atheromatous plaques. Circulation. 1999;99(19):2503–9.
Virmani R, Kolodgie FD, Burke AP, Finn AV, Gold HK, Tulenko TN, et al. Atherosclerotic plaque progression and vulnerability to rupture: angiogenesis as a source of intraplaque hemorrhage. Arterioscler Thromb Vasc Biol. 2005;25(10):2054–61.
Takaya N, Yuan C, Chu B, Saam T, Polissar NL, Jarvik GP, et al. Presence of intraplaque hemorrhage stimulates progression of carotid atherosclerotic plaques: a high-resolution magnetic resonance imaging study. Circulation. 2005;111(21):2768–75.
Bourantas CV, Garcia-Garcia HM, Farooq V, Maehara A, Xu K, Genereux P, et al. Clinical and angiographic characteristics of patients likely to have vulnerable plaques: analysis from the PROSPECT study. JACC Cardiovasc Imaging. 2013;6(12):1263–72.
Fleg JL, Stone GW, Fayad ZA, Granada JF, Hatsukami TS, Kolodgie FD, et al. Detection of high-risk atherosclerotic plaque: report of the NHLBI Working Group on current status and future directions. JACC Cardiovasc Imaging. 2012;5(9):941–55.
Stone GW, Maehara A, Lansky AJ, de Bruyne B, Cristea E, Mintz GS, et al. A prospective natural-history study of coronary atherosclerosis. N Engl J Med. 2011;364(3):226–35.
Calvert PA, Obaid DR, O’Sullivan M, Shapiro LM, McNab D, Densem CG, et al. Association between IVUS findings and adverse outcomes in patients with coronary artery disease: the VIVA (VH-IVUS in Vulnerable Atherosclerosis) Study. JACC Cardiovasc Imaging. 2011;4(8):894–901.
Kume T, Akasaka T, Kawamoto T, Okura H, Watanabe N, Toyota E, et al. Measurement of the thickness of the fibrous cap by optical coherence tomography. Am Heart J. 2006;152(4):755.e1–4.
Narula J, Nakano M, Virmani R, Kolodgie FD, Petersen R, Newcomb R, et al. Histopathologic characteristics of atherosclerotic coronary disease and implications of the findings for the invasive and noninvasive detection of vulnerable plaques. J Am Coll Cardiol. 2013;61(10):1041–51.
Tian J, Ren X, Vergallo R, Xing L, Yu H, Jia H, et al. Distinct morphological features of ruptured culprit plaque for acute coronary events compared to those with silent rupture and thin-cap fibroatheroma: a combined optical coherence tomography and intravascular ultrasound study. J Am Coll Cardiol. 2014;63(21):2209–16.
Tian J, Dauerman H, Toma C, Samady H, Itoh T, Kuramitsu S, et al. Prevalence and characteristics of TCFA and degree of coronary artery stenosis: an OCT, IVUS, and angiographic study. J Am Coll Cardiol. 2014;64(7):672–80.
Sinclair H, Bourantas C, Bagnall A, Mintz GS, Kunadian V. OCT for the identification of vulnerable plaque in acute coronary syndrome. JACC Cardiovasc Imaging. 2015;8(2):198–209.
Waksman R, Torguson R, Spad MA, Garcia-Garcia H, Ware J, Wang R, et al. The Lipid-Rich Plaque Study of vulnerable plaques and vulnerable patients: study design and rationale. Am Heart J. 2017;192:98–104.
Waksman R. Assessment of coronary near-infrared spectroscopy imaging to detect vulnerable plaques and vulnerable patients. Presented at: TCT 2018. September 24, 2018, San Diego, CA.
Stone GW, Maehara A, Mintz GS. The reality of vulnerable plaque detection. JACC Cardiovasc Imaging. 2011;4(8):902–4.
Arbab-Zadeh A, Fuster V. The myth of the “vulnerable plaque”: transitioning from a focus on individual lesions to atherosclerotic disease burden for coronary artery disease risk assessment. J Am Coll Cardiol. 2015;65(8):846–55.
Mann J, Davies MJ. Mechanisms of progression in native coronary artery disease: role of healed plaque disruption. Heart. 1999;82(3):265–8.
Burke AP, Kolodgie FD, Farb A, Weber DK, Malcom GT, Smialek J, et al. Healed plaque ruptures and sudden coronary death: evidence that subclinical rupture has a role in plaque progression. Circulation. 2001;103(7):934–40.
Kubo T, Maehara A, Mintz GS, Doi H, Tsujita K, Choi SY, et al. The dynamic nature of coronary artery lesion morphology assessed by serial virtual histology intravascular ultrasound tissue characterization. J Am Coll Cardiol. 2010;55(15):1590–7.
Naghavi M, Libby P, Falk E, Casscells SW, Litovsky S, Rumberger J, et al. From vulnerable plaque to vulnerable patient: a call for new definitions and risk assessment strategies: part I. Circulation. 2003;108(14):1664–72.
Naghavi M, Libby P, Falk E, Casscells SW, Litovsky S, Rumberger J, et al. From vulnerable plaque to vulnerable patient: a call for new definitions and risk assessment strategies: part II. Circulation. 2003;108(15):1772–8.
Manoharan G, Ntalianis A, Muller O, Hamilos M, Sarno G, Melikian N, et al. Severity of coronary arterial stenoses responsible for acute coronary syndromes. Am J Cardiol. 2009;103(9):1183–8.
Glaser R, Selzer F, Faxon DP, Laskey WK, Cohen HA, Slater J, et al. Clinical progression of incidental, asymptomatic lesions discovered during culprit vessel coronary intervention. Circulation. 2005;111(2):143–9.
Yokoya K, Takatsu H, Suzuki T, Hosokawa H, Ojio S, Matsubara T, et al. Process of progression of coronary artery lesions from mild or moderate stenosis to moderate or severe stenosis: a study based on four serial coronary arteriograms per year. Circulation. 1999;100(9):903–9.
Glagov S, Weisenberg E, Zarins CK, Stankunavicius R, Kolettis GJ. Compensatory enlargement of human atherosclerotic coronary arteries. N Engl J Med. 1987;316(22):1371–5.
Kolodgie FD, Gold HK, Burke AP, Fowler DR, Kruth HS, Weber DK, et al. Intraplaque hemorrhage and progression of coronary atheroma. N Engl J Med. 2003;349(24):2316–25.
Kumamoto M, Nakashima Y, Sueishi K. Intimal neovascularization in human coronary atherosclerosis: its origin and pathophysiological significance. Hum Pathol. 1995;26(4):450–6.
•• Ahmadi A, Leipsic J, Blankstein R, Taylor C, Hecht H, Stone GW, et al. Do plaques rapidly progress prior to myocardial infarction? The interplay between plaque vulnerability and progression. Circ Res. 2015;117(1):99–104 Ahmadi et al. challenge the perception in the cardiology community that most acute coronary events arise from ruptures of mildly stenotic plaques. They clarify that rapid plaque progression of moderately severe vulnerable plaques is the critical step before MI in most cases and provide evidence from serial angiographic as well as imaging studies. The authors propose the detection of the rate of plaque progression as an additional factor to identify vulnerable plaques with an increased potential for adverse outcomes.
Goff DC Jr, Lloyd-Jones DM, Bennett G, Coady S, D’Agostino RB, Gibbons R, et al. 2013 ACC/AHA guideline on the assessment of cardiovascular risk: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation. 2014;129(25 Suppl 2):S49–73.
Wilson PW, D’Agostino RB, Levy D, Belanger AM, Silbershatz H, Kannel WB. Prediction of coronary heart disease using risk factor categories. Circulation. 1998;97(18):1837–47.
Conroy RM, Pyorala K, Fitzgerald AP, Sans S, Menotti A, De Backer G, et al. Estimation of ten-year risk of fatal cardiovascular disease in Europe: the SCORE project. Eur Heart J. 2003;24(11):987–1003.
Goff DC Jr, Lloyd-Jones DM, Bennett G, Coady S, D’Agostino RB Sr, Gibbons R, et al. 2013 ACC/AHA guideline on the assessment of cardiovascular risk: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2014;63(25 Pt B):2935–59.
Wald NJ, Morris JK, Rish S. The efficacy of combining several risk factors as a screening test. J Med Screen. 2005;12(4):197–201.
Lauer MS. Primary prevention of atherosclerotic cardiovascular disease: the high public burden of low individual risk. Jama. 2007;297(12):1376–8.
•• Al Rifai M, Cainzos-Achirica M, Kianoush S, Mirbolouk M, Peng A, Comin-Colet J, et al. Coronary Artery Calcium: Recommendations for Risk Assessment in Cardiovascular Prevention Guidelines. Curr Treat Options Cardiovasc Med. 2018;20(11):89 Al Rifai et al. evaluate the coronary artery calcium (CAC) score as a biomarker for advanced atherosclerotic cardiovascular disease (ASCVD) risk assessment and demonstrate a clear advantage of CAC compared with traditional and non-traditional cardiovascular risk factors. The authors recommend CAC especially for refining risk assessment among intermediate risk patients.
• Takayama Y, Yasuda Y, Suzuki S, Shibata Y, Tatami Y, Shibata K, et al. Relationship between abdominal aortic and coronary artery calcification as detected by computed tomography in chronic kidney disease patients. Heart Vessel. 2016;31(7):1030–7 In this study, Takayama et al. investigate the relationship between abdominal aortic calcification and coronary artery calcification in chronic kidney disease patients by means of a non-contrast computed tomography scan. The aortic calcification index (ACI) was independently associated with severe CAC score and the addition of ACI to traditional risk factors significantly improved the predictive ability of severe CAC score. The authors suggest ACI as a screening tool in clinical practice.
Zweig BM, Sheth M, Simpson S, Al-Mallah MH. Association of abdominal aortic calcium with coronary artery calcium and obstructive coronary artery disease: a pilot study. Int J Card Imaging. 2012;28(2):399–404.
Allison MA, Hsi S, Wassel CL, Morgan C, Ix JH, Wright CM, et al. Calcified atherosclerosis in different vascular beds and the risk of mortality. Arterioscler Thromb Vasc Biol. 2012;32(1):140–6.
• O’Connor SD, Graffy PM, Zea R, Pickhardt PJ. Does nonenhanced CT-based quantification of abdominal aortic calcification outperform the Framingham risk score in predicting cardiovascular events in asymptomatic adults? Radiology. 2019;290(1):108–15 O’Connor et al. demonstrate that non-contrast enhanced CT-based measurement of abdominal aortic calcification could strongly predict future cardiovascular events, outperforming the Framingham risk score, in 829 generally healthy asymptomatic adults in a 10-year follow-up.
Puchner SB, Liu T, Mayrhofer T, Truong QA, Lee H, Fleg JL, et al. High-risk plaque detected on coronary CT angiography predicts acute coronary syndromes independent of significant stenosis in acute chest pain: results from the ROMICAT-II trial. J Am Coll Cardiol. 2014;64(7):684–92.
• Reinhardt SW, Lin CJ, Novak E, Brown DL. Noninvasive cardiac testing vs clinical evaluation alone in acute chest pain: a secondary analysis of the ROMICAT-II randomized clinical trial. JAMA Intern Med. 2018;178(2):212–9 This retrospective analysis of data from ROMICAT II included 1000 patients who presented with chest pain and compared the outcomes after clinical evaluation and coronary computed tomographic angiography vs. clinical evaluation alone. Reinhardt et al. revealed a longer length of stay, more downstream testing, more radiation exposure, and greater cost without an improvement in clinical outcomes if patients presented with acute chest pain, but had negative biomarkers and a non-ischemic ECG result. This study underlines the importance of a common sense use of imaging methods.
•• Lin JS, Evans CV, Johnson E, Redmond N, Coppola EL, Smith N, et al. Jama. 2018;320(3):281–97 This systematic review of 43 studies including 267,244 patients evaluates non-traditional risk factors (ankle-brachial index, high-sensitivity C-reactive protein level, and coronary artery calcium score) in cardiovascular risk assessment. Lin et al. demonstrate a lack of direct evidence from adequately powered trials whether the addition of non-traditional risk factors will improve patient health outcomes. Thus, there is clearly a need for further research in this field, especially with regard to the finding of coronary artery calcium.
Grundy SM, Stone NJ, Bailey AL, Beam C, Birtcher KK, Blumenthal RS, et al. AHA/ACC/AACVPR/AAPA/ABC/ACPM/ADA/AGS/APhA/ASPC/NLA/PCNA guideline on the management of blood cholesterol. Circulation. 2018. https://doi.org/10.1161/CIR.0000000000000625.
Baigent C, Keech A, Kearney PM, Blackwell L, Buck G, Pollicino C, et al. Efficacy and safety of cholesterol-lowering treatment: prospective meta-analysis of data from 90,056 participants in 14 randomised trials of statins. Lancet. 2005;366(9493):1267–78.
Montalescot G, Sechtem U, Achenbach S, Andreotti F, Arden C, Budaj A, et al. 2013 ESC guidelines on the management of stable coronary artery disease: the task force on the management of stable coronary artery disease of the European Society of Cardiology. Eur Heart J. 2013;34(38):2949–3003.
O’Gara PT, Kushner FG, Ascheim DD, Casey DE Jr, Chung MK, de Lemos JA, et al. 2013 ACCF/AHA guideline for the management of ST-elevation myocardial infarction: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. Circulation. 2013;127(4):e362–425.
Ray KK, Cannon CP. The potential relevance of the multiple lipid-independent (pleiotropic) effects of statins in the management of acute coronary syndromes. J Am Coll Cardiol. 2005;46(8):1425–33.
Kataoka Y, Wolski K, Balog C, Uno K, Puri R, Tuzcu EM, et al. Progression of coronary atherosclerosis in stable patients with ultrasonic features of high-risk plaques. Eur Heart J Cardiovasc Imaging. 2014;15(9):1035–41.
•• Lee SE, Chang HJ, Sung JM, Park HB, Heo R, Rizvi A, et al. Effects of statins on coronary atherosclerotic plaques: the PARADIGM study. JACC Cardiovasc Imaging. 2018;11(10):1475–84 The PARADIGM study demonstrates a slower progression of overall coronary atherosclerosis volume and a reduction of high-risk plaque features in 2,496 coronary artery lesions from patients on statins as compared with 1,079 coronary artery lesions from statin-naïve patients. This emphasizes the benefit of a statin therapy upon vulnerable plaques.
Baigent C, Blackwell L, Emberson J, Holland LE, Reith C, Bhala N, et al. Efficacy and safety of more intensive lowering of LDL cholesterol: a meta-analysis of data from 170,000 participants in 26 randomised trials. Lancet. 2010;376(9753):1670–81.
Kataoka Y, Puri R, Hammadah M, Duggal B, Uno K, Kapadia SR, et al. Frequency-domain optical coherence tomographic analysis of plaque microstructures at nonculprit narrowings in patients receiving potent statin therapy. Am J Cardiol. 2014;114(4):549–54.
Giugliano RP, Sabatine MS. Are PCSK9 inhibitors the next breakthrough in the cardiovascular field? J Am Coll Cardiol. 2015;65(24):2638–51.
• Robinson JG, Farnier M, Krempf M, Bergeron J, Luc G, Averna M, et al. Efficacy and safety of alirocumab in reducing lipids and cardiovascular events. N Engl J Med. 2015;372(16):1489–99 This breakthrough study demonstrated the safety and efficacy of alirocumab in addition to a statin therapy and paved the way for a more efficient therapeutic reduction of low-density lipoprotein (LDL) cholesterol levels.
Sabatine MS, Giugliano RP, Keech AC, Honarpour N, Wiviott SD, Murphy SA, et al. Evolocumab and clinical outcomes in patients with cardiovascular disease. N Engl J Med. 2017;376(18):1713–22 The Fourier study demonstrated that evolocumab could efficiently lower LDL cholesterol levels and to reduce the risk of cardiovascular events.
• Lloyd-Jones DM, Morris PB, Ballantyne CM, Birtcher KK, Daly DD Jr, DePalma SM, et al. Focused Update of the 2016 ACC Expert Consensus Decision Pathway on the role of non-statin therapies for LDL-cholesterol lowering in the management of atherosclerotic cardiovascular disease risk: a report of the American College of Cardiology Task Force on Expert Consensus Decision Pathways. J Am Coll Cardiol. 2017;70(14):1785–822 Since PCSK9 inhibitors have proven beneficial in cardiovascular outcomes trials in patients with CAD, this focused update of the expert consensus decision pathway on the role of non-statin therapies for LDL cholesterol lowering provides guidance for clinicians on decision making regarding the use of ezetimibe and PCSK9 inhibitors.
Puri R, Nissen SE, Somaratne R, Cho L, Kastelein JJ, Ballantyne CM, et al. Impact of PCSK9 inhibition on coronary atheroma progression: rationale and design of global assessment of plaque regression with a PCSK9 antibody as measured by intravascular ultrasound (GLAGOV). Am Heart J. 2016;176:83–92.
•• Nicholls SJ, Puri R, Anderson T, Ballantyne CM, Cho L, Kastelein JJP, et al. Effect of evolocumab on coronary plaque composition. J Am Coll Cardiol. 2018;72(17):2012–21 Nicholls et al. investigated in GLAGOV if the addition of evolocumab to a statin would alter the plaque composition, as assessed by IVUS in 968 patients with CAD. Whereas statins have been shown to reduce high-risk plaque features, the addition of evolocumab to a statin did not produce differential changes in plaque composition. However, the addition of evolocumab induced plaque regression in a greater percentage of patients and thus might be a potential therapeutic option for patients harboring vulnerable plaques.
Ridker PM, Cushman M, Stampfer MJ, Tracy RP, Hennekens CH. Inflammation, aspirin, and the risk of cardiovascular disease in apparently healthy men. N Engl J Med. 1997;336(14):973–9.
Ross R. Atherosclerosis--an inflammatory disease. N Engl J Med. 1999;340(2):115–26.
• Ridker PM, Everett BM, Thuren T, MacFadyen JG, Chang WH, Ballantyne C, et al. Antiinflammatory therapy with canakinumab for atherosclerotic disease. N Engl J Med. 2017;377(12):1119–31 This randomized, double-blind trial investigates canakinumab, a monoclonal antibody targeting interleukin-1β. Only the 150-mg dose, but not the 50- and 300-mg doses, met the threshold for statistical significance for the primary and secondary end points. Canakinumab was associated with a higher incidence of fatal infection than was placebo. This study sought to investigate whether non-lipid-lowering targets might be of potential therapeutic benefit for lowering atherosclerotic risk.
• Ridker PM, Everett BM, Pradhan A, MacFadyen JG, Solomon DH, Zaharris E, et al. Low-dose methotrexate for the prevention of atherosclerotic events. N Engl J Med. 2018;380:752–62. https://doi.org/10.1056/NEJMoa1809798. In this study, low-dose methotrexate for the prevention of atherosclerotic events failed to reduce cardiovascular events versus placebo. This emphasizes that although lowering inflammation might seen like a logical pathway to decrease risk from vulnerable plaques, the efficacy of such an approach has yet to be shown.
Patel MR, Calhoon JH, Dehmer GJ, Grantham JA, Maddox TM, Maron DJ, et al. ACC/AATS/AHA/ASE/ASNC/SCAI/SCCT/STS 2016 Appropriate Use Criteria for Coronary Revascularization in Patients With Acute Coronary Syndromes: a report of the American College of Cardiology Appropriate Use Criteria Task Force, American Association for Thoracic Surgery, American Heart Association, American Society of Echocardiography, American Society of Nuclear Cardiology, Society for Cardiovascular Angiography and Interventions, Society of Cardiovascular Computed Tomography, and the Society of Thoracic Surgeons. J Am Coll Cardiol. 2017;69(5):570–91.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
CVPath Institute has received institutional research support from 480 Biomedical, Abbott Vascular, ART, BioSensors International, Biotronik, Boston Scientific, Celonova, Claret Medical, Cook Medical, Cordis, Edwards Lifesciences, Medtronic, MicroPort, MicroVention, OrbusNeich, ReCore, SINO Medical Technology, Spectranetics, Surmodics, Terumo Corporation, W.L. Gore and Xeltis. R.V. has received honoraria from 480 Biomedical, Abbott Vascular, Boston Scientific, Cook Medical, Lutonix, Medtronic, Terumo Corporation and W.L. Gore; and is a consultant for 480 Biomedical, Abbott Vascular, Medtronic, and W.L. Gore. A.V. Finn has received honoraria from Boston Scientific, Abbott Vascular, Amgen, and CeloNova. A.C. receives research grants from University Hospital RWTH Aachen.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection on Secondary Prevention and Intervention
Rights and permissions
About this article
Cite this article
Cornelissen, A., Jinnouchi, H., Sakamoto, A. et al. Evaluation and Management of the Vulnerable Plaque. Curr Cardiovasc Risk Rep 13, 14 (2019). https://doi.org/10.1007/s12170-019-0606-0
Published:
DOI: https://doi.org/10.1007/s12170-019-0606-0