Abstract
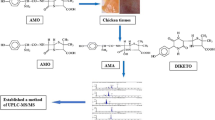
An ultra-performance liquid chromatography-electrospray ionization tandem triple quadrupole mass spectrometry (UPLC-ESI/MS/MS) method was developed for the detection of piperazine in chicken muscle. Following extraction and purification, the chicken muscle extract was injected into the UPLC system and analyzed. Piperazine detection was performed on a triple-quadrupole mass spectrometer with the ESI interface operating in positive mode. The most sensitive mass transition from the precursor ion to the product ion was 87.1 → 44.1 for piperazine. The coefficient (R 2) of piperazine was 0.9995 in the range of 1–200 μg/kg. The recovery and relative standard deviation (RSD) ranged from 102.93 to 111.46% and from 4.57 to 5.28% at 50.0, 100.0 and 200.0 μg/kg adding levels, respectively. Limits of detection (LODs) and limits of quantification (LOQs) were 0.4 and 1.0 μg/kg, respectively. This method was fully validated based on its specificity, sensitivity, linearity, precision, accuracy, matrix effect, and stability results. Compared to other researches, this new method not only omitted the derivative process, which simplify the pre-processing, but also proved a better sensitivity based on its low LOD and LOQ values.



Similar content being viewed by others
References
Bond GR (1960) Determination of piperazine as piperazine diacetate. Anal Chem 32:1332–1334
Becker M, Zittlau E, Petz M (2004) Residue analysis of 15 penicillins and cephalosporins in bovine muscle, kidney and milk by liquid chromatography–tandem mass spectrometry. Anal Chim Acta 520:19–32
Bonfiglio R, King RC, Olah TV, Merkle K (1999) The effects of sample preparation methods on the variability of the electrospray ionization response for model drug compound. Rapid Commun Mass Sp 13:1175–1185
Commission Decision (2002) Commission decision of 2002/657/EC implementing council directive 96/23/EC concerning the performance of analytical methods and the interpretation of results (2002/657/EC). Official Journal of European Communities L221:8–36
Dessouky YM, Ismaiel SA (1974) Colorimetric determination of piperazine in pharmaceutical formulations. Analyst 99:482–486
Lacorte S, Fernandez-Alba A (2006) Time of flight mass spectrometry applied to the liquid chromatographic analysis of pesticides in water and food. Mass Spec Rev 25:866–880
Lin H, Tian Y, Zhang Z, Wu L, Chen Y (2010) Quantification of piperazine phosphate in human plasma by high-performance liquid chromatography-electrospray ionization tandem mass spectrometry employing precolumn derivatization with dansyl chloride. Anal Chim Acta 664:40–48
Matuszewski BK, Constanzer ML, Chavez-Eng CM (2003) Strategies for the assessment of matrix effect in quantitative bioanalytical methods based on HPLC-MS/MS. Anal Chem 75:3019–3030
Mc Clintic C, Remick DM, Peterson JA, Risley DS (2003) Novel method for the determination of piperazine in pharmaceutical drug substances using hydrophilic interaction chromatography and evaporative light scattering detection. J Liq Chromatogr R T 26:3093–3104
Muralikrishna U, Babu KS, Krishnamurthy M (1990) A simple spectrophotometric determination of some chlorobenzoquinones with morpholine, thiomorpholine and piperazine. Talanta 37:353–355
Navaneeswari R, Reddy PR (2012) Analytical method for piperazine in an active pharmaceutical ingredient using chemical derivatization and HPLC-UV. J Chem Pharml Res 4:2854–2859
Park JA, Zhang D, Kim DS, Kim SK, Cho SH, Jeong D (2015) Development of a high-performance liquid chromatography with fluorescence detection method for quantification of piperazine in animal products by using precolumn derivatization. Food Chem 196:1331–1337
Skarping G, Bellander T, Mathiasson L (1986) Determination of piperazine in working atmosphere and in human urine using derivatization and capillary gas chromatography with nitrogen- and mass-selective detection. J Chromatogr B 370:245–258
Senthilraja M (2006) Colorimetric method for the determination of piperazine in pharmaceutical formulations. Indian J Pharm Sci 68:843
The Japan Food Chemical Research Foundation (2015) Maximum residue limits (MRLs) list of agricultural chemicals in foods
U.S. Department of Health and Human Services, Food and Drug Administration (2001) Center for Drug Evaluation and Research, Center for Veterinary Medicine, Bioanalytical Method Validation, Guidance for Industry, p 1–22
U.S. Food and Drug Administration (2014) CFR–Code of Federal regulations title 21 part 556 tolerances for residues of new animal drugs in food
Acknowledgements
This work was Supported by the China Agriculture Research System (CARS-42-G23), the National Natural Science Foundation of China (31302009), the National Science and Technology Pillar Program during the Twelfth Five-year Plan Period of China (2014BAD13B02), the Priority Academic Program Development (PAPD) of Jiangsu Higher Education Institutions, and the New Century Talent Project of Yangzhou University.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
Kaizhou Xie declares that he has no conflict of interest. Ya’nan Liu declares that he has no conflict of interest. Lirui Sun declares that he has no conflict of interest. Maoda Pang declares that he has no conflict of interest. Xing Xie declares that he has no conflict of interest. Qiang Gao declares that he has no conflict of interest. Bo Wang declares that he has no conflict of interest. Yangyang Zhang declares that he has no conflict of interest. Ran Wang declares that he has no conflict of interest. Genxi Zhang declares that he has no conflict of interest. Guojun Dai declares that he has no conflict of interest. Jinyu Wang declares that he has no conflict of interest.
Ethical Approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Informed Consent
Not applicable.
Rights and permissions
About this article
Cite this article
Xie, K., Liu, Y., Sun, L. et al. Quantification of Piperazine in Chicken Muscle by Ultra-Performance Liquid Chromatography-Electrospray Ionization Tandem Mass Spectrometry. Food Anal. Methods 10, 1736–1744 (2017). https://doi.org/10.1007/s12161-016-0717-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12161-016-0717-x