Abstract
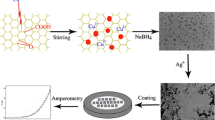
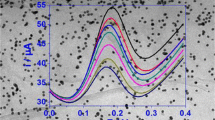
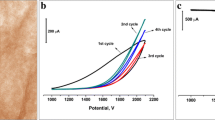
Nitrogen-doped graphene oxide (NGO) was synthesized via pyrolysis of graphene oxide and urea and was characterized by transmission electron microscopy (TEM) and X-ray photoelectron spectroscopy (XPS). An electrochemically reduced nitrogen-doped graphene oxide-modified glassy carbon electrode (ERNGO/GCE) was developed for the determination of carbendazim (CBZ) in food samples. The surface morphology of the modified electrode was characterized by scanning electron microscopy (SEM). Cyclic voltammetry and electrochemical impedance spectroscopy were employed to demonstrate the large electrode surface and fast electron transfer of the ERNGO/GCE. Electrochemical behaviors of CBZ at different electrodes were studied by voltammetry. Experimental results showed that the ERNGO/GCE achieved better performance for the electrochemical oxidation of CBZ than either the bare glassy carbon electrode (GCE) or the nitrogen-doped graphene oxide-modified GCE (NGO/GCE). Under optimized conditions, the ERNGO/GCE exhibited a wide linearity of 5.0~850 μg/L with a detection limit of 1.0 μg/L (signal-to-noise ratio = 3). Application of our proposed method in food products was shown to be practical and reliable.









Similar content being viewed by others
References
Chen D, Feng H, Li J (2012) Graphene oxide: preparation, functionalization, and electrochemical applications. Chem Rev 112:6027–6053
Chen H et al (2015a) Determination of thiophanate-methyl and carbendazim in rapeseed by solid–phase extraction and ultra–high performance chromatography with photodiode array detection. Instrum Sci Technol 43:511–523
Chen M, Zhao Z, Lan X, Chen Y, Zhang L, Ji R, Wang L (2015b) Determination of carbendazim and metiram pesticides residues in reapeseed and peanut oils by fluorescence spectrophotometry. Measurement 73:313–317
Devi PA, Paramasivam M, Prakasam V (2015) Degradation pattern and risk assessment of carbendazim and mancozeb in mango fruits. Environ Monit Assess 187:4142
Du H, Ye J, Zhang J, Huang X, Yu C (2011) A voltammetric sensor based on graphene-modified electrode for simultaneous determination of catechol and hydroquinone. J Electroanal Chem 650:209–213
Du M, Sun J, Chang J, Yang F, Shi L, Gao L (2014) Synthesis of nitrogen-doped reduced graphene oxide directly from nitrogen-doped graphene oxide as a high-performance lithium ion battery anode. RSC Adv 4:42412–42417
Farag A, Ebrahim H, Elmazoudy R, Kadous E (2011) Developmental toxicity of fungicide carbendazim in female mice. Birth Defects Res Part B 92:122–130
França RF, Oliveira HPMD, Pedrosa VA, Codognoto L (2012) Electroanalytical determination of carbendazim and fenamiphos in natural waters using a diamond electrode. Diam Relat Mater 27–28:54–59
Kuila T, Bose S, Mishra AK, Khanra P, Kim NH, Lee JH (2012) Chemical functionalization of graphene and its applications. Prog Mater Sci 57:1061–1110
Laviron E (1974) Adsorption, autoinhibition and autocatalysis in polarography and in linear potential sweep voltammetry. J Electroanal Chem 52:355–393
Lewandowska A, Walorczyk S (2010) Carbendazim residues in the soil and their bioavailability to plants in four successive harvests. Pol J Environ Stud 19:757–761
Li F, Gan S, Han D, Niu L (2015) Graphene-based nanohybrids for advanced electrochemical sensing. Electroanal 27:2098–2115
Liao S, Xie Z (2006) Flow-injection chemiluminescence study of luminal-hydrogen peroxide-carbendazim system. Spectrosc Lett 39:473–485
Lima T, Silva HTD, Labuto G, Simões FR, Codognoto L (2016) An experimental design for simultaneous determination of carbendazim and fenamiphos by electrochemical method. Electroanal 28:817–822
Liu L, Gou Y, Gao X, Zhang P, Chen W, Feng S, Hu F, Li Y (2014) Electrochemically reduced graphene oxide-based electrochemical sensor for the sensitive determination of ferulic acid in A. sinensis and biological samples. Mat Sci Eng C 42:227–223
Llorent-Martínez EJ, Alcántara-Durán J, Ruiz-Medina A, Ortega-Barrales P (2013) Determination of carbendazim in food products using a sequential injection analysis optosensor. Food Anal Methods 6:1278–1283
Luo S, Wu Y, Gou H (2013) A voltammetric sensor based on GO–MWNTs hybrid nanomaterial-modified electrode for determination of carbendazim in soil and water samples. Ionics 19:673–680
Manisankar P, Selvanathan G, Vedhi C (2006) Determination of pesticides using heteropolyacid montmorillonite clay-modified electrode with surfactant. Talanta 68:686–692
Maximiano EM, Lima F, Cardoso CAL, Arruda GJ (2016) Incorporation of thermally activated zeolite into carbon paste electrodes for voltammetric detection of carbendazim traces in milk samples. J Appl Electrochem 46:713–723
Novoselov KS, Geim AK, Morozov SV, Jiang D, Zhang Y, Dubonos SV, Grigorieva IV, Firsov A (2004) Electric field effect in atomically thin carbon films. Science 306:666–669
Noyrod P, Chailapakul O, Wonsawat W, Chuanuwatanakul S (2014) The simultaneous determination of isoproturon and carbendazim pesticides by single drop analysis using a graphene-based electrochemical sensor. J Electroanal Chem 719:54–59
Petroni JM, Lucca BG, Fogliato DK, Ferreira VS (2016) Sensitive approach for voltammetric determination of carbendazim based on the use of an anionic surfactant. Electroanal 28:1362–1369
Pourreza N, Rastegarzadeh S, Larki A (2015) Determination of fungicide carbendazim in water and soil samples using dispersive liquid-liquid microextraction and microvolume UV–vis spectrophotometry. Talanta 134:24–29
Qiao W, Li Y, Wang L, Li G, Li J, Ye B (2015) Electrochemical behavior of daphnetin and its sensitive determination based on electrochemically reduced graphene oxide modified electrode. J Electroanal Chem 749:68–74
Qin X, Xu Y, Sun Y, Zhao L, Wang L, Sun Y, Liang X (2016) Determination of carbendazim and diethofencarb in cotton and soil by high-performance liquid chromatography. Anal Lett 49:1631–1639
Rama EM, Bortolan S, Vieira ML, Moreira EG (2014) Reproductive and possible hormonal effects of carbendazim. Regul Toxicol Pharmacol 69:476–486
Razzino CA, Sgobbi LF, Canevari TC, Cancino J, Machado SAS (2015) Sensitive determination of carbendazim in orange juice by electrode modified with hybrid material. Food Chem 170:360–365
Shao Y et al (2010) Nitrogen-doped graphene and its electrochemical applications. J Mater Chem 20:7491–7496
Strickland AD, Batt CA (2009) Detection of carbendazim by surface-enhanced raman scattering using cyclodextrin inclusion complexes on gold nanorods. Anal Chem 81:2895–2903
Subhani Q, Huang ZP, Zhu ZY, Zhu Y (2013) Simultaneous determination of imidacloprid and carbendazim in water samples by ion chromatography with fluorescence detector and post-column photochemical reactor. Talanta 116:127–132
Sun D, Wang S (2015) Highly sensitive electrochemical sensor for paeonol using NMP-exfoliated grapheme-modified electrode. Ionics 21:1–6
Thomidis T, Michailides T, Exadaktylou E (2009) Contribution of pathogens to peach fruit rot in northern Greece and their sensitivity to iprodione, carbendazim, thiophanate-methyl and tebuconazole fungicides. J Phytopathol 157:194–200
Usachov D et al (2011) Nitrogen-doped graphene: efficient growth, structure, and electronic properties. Nano Lett 11:5401–5407
Wang X, Sun G, Routh P, Kim DH, Huang W, Chen P (2014) Heteroatom-doped graphene materials: syntheses, properties and applications. Chem Soc Rev 43:7067–7098
Wang Y, Shao Y, Matson DW, Li J, Lin Y (2010) Nitrogen-doped graphene and its application in electrochemical biosensing. ACS Nano 4:1790–1798
Wei T, Dai Z, Lin Y, Du D (2016) Electrochemical immunoassays based on graphene: a review. Electroanal 28:4–12
Ya Y, Wang T, Xie L, Zhu J, Tang L, Ning D, Yan F (2015) Highly sensitive electrochemical sensor based on pyrrolidinium ionic liquid modified ordered mesoporous carbon paste electrode for determination of carbendazim. Anal Methods 7:1493–1498
Yao Y, Wen Y, Zhang L, Wang Z, Zhang H, Xu J (2014) Electrochemical recognition and trace-level detection of bactericide carbendazim using carboxylic group functionalized poly (3,4-ethylenedioxythiophene) mimic electrode. Anal Chim Acta 831:38–49
Zhou N, Li JH, Chen H, Liao CY, Chen LX (2013) A functional grapheneoxide-ionic liquid composites–gold nanoparticle sensing platform for ultrasensitive electrochemical detection of Hg2+. Analyst 138:1091–1097
Acknowledgments
This work was financially supported by the Natural Science Foundation of Guangxi for Youth (No. 2015GXNSFBA139037), the Fundamental Research Funds for the Guangxi Academy of Agricultural Sciences (2015YT94), and the Achievement Transformation Project of Guangxi Academy of Agricultural Sciences (2016020).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
This study was funded by the Natural Science Foundation of Guangxi for Youth (No. 2015GXNSFBA139037), the Fundamental Research Funds for the Guangxi Academy of Agricultural Sciences (No. 2015YT94), and the Achievement Transformation Project of Guangxi Academy of Agricultural Sciences (No. 2016020).
Conflict of Interest
Yu Ya declares that he has no conflict of interest. Cuiwen Jiang declares that he has no conflict of interest. Leixing Mo declares that he has no conflict of interest. Tao Li declares that he has no conflict of interest. Liping Xie declares that he has no conflict of interest. Jie He declares that he has no conflict of interest. Li Tang declares that he has no conflict of interest. Dejiao Ning declares that he has no conflict of interest. Feiyan Yan declares that he has no conflict of interest.
Ethical Approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Informed Consent
Not applicable.
Rights and permissions
About this article
Cite this article
Ya, Y., Jiang, C., Mo, L. et al. Electrochemical Determination of Carbendazim in Food Samples Using an Electrochemically Reduced Nitrogen-Doped Graphene Oxide-Modified Glassy Carbon Electrode. Food Anal. Methods 10, 1479–1487 (2017). https://doi.org/10.1007/s12161-016-0708-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12161-016-0708-y