Abstract
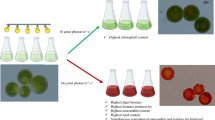
Biorefining of Haematococcus pluvialis can be employed through the integration of different bio-product production from the same feedstock. In this regard, the present study evaluated the potential of utilizing microalga H. pluvialis to produce biodiesel without being constricted to astaxanthin under nitrogen deprivation. Hence, the impact of nitrogen limitation was investigated on the growth parameters, photosynthetic pigments, astaxanthin, and biochemical composition as well as the fatty acid (FA) profile of H. pluvialis. Finally, the physical/chemical features of biodiesel produced from H. pluvialis were assessed. Nitrogen deprivation (0 mg /L) decreased the cell number (/mL), biomass (g/ L), cell size, growth rate (µ), and biomass productivity (g/ L/ d) of H. pluvialis during 40 days of the experiment. Additionally, a remarkable reduction was found in chlorophyll content (pg/cell) under nitrogen deprivation over the culture period, whilst astaxanthin (µg/cell) was found to be four times higher on the 40th day. Following treatment for 40 days, the carbohydrate and protein contents of the control culture (nitrogen-rich culture) were highest, while the lipid content of H. pluvialis did not change significantly under nitrogen stress. Besides, saturated fatty acids (SFAs) accounted for 75% of the total FAs under nitrogen starvation. Hence, the high SFAs level and the lowest level of C18 FAs determined the suitability of H. pluvialis grown in nitrogen-free culture for the production of biodiesel. Accordingly, cellular stresses, such as nitrogen limitation, increase the production of astaxanthin in H. pluvialis simultaneously by changes in the quality and quantity of biochemical composition, such as FAs.






Similar content being viewed by others
Data Availability
All datasets generated for this study are included in the manuscript.
References
Saidur R, BoroumandJazi G, Mekhilef S, Mohammed HA (2012) A review on exergy analysis of biomass based fuels. Renew Sustain Energy Rev 16:1217–1222. https://doi.org/10.1016/j.rser.2011.07.076
Chandra R, Iqbal HMN, Vishal G, Lee HS, Nagra S (2019) Algal biorefinery: a sustainable approach to valorize algal-based biomass towards multiple product recovery. Bioresour Technol 278:346–359. https://doi.org/10.1016/j.biortech.2019.01.104
Ho S-H, Huang S-W, Chen C-Y, Hasunuma T, Kondo A, Chang JS (2013) Bioethanol production using carbohydrate-rich microalgae biomass as feedstock. Bioresour Technol 135:191–198. https://doi.org/10.1016/j.biortech.2012.10.015
Behera B, Selvam SM, Paramasivan B (2022) Research trends and market opportunities of microalgal biorefinery technologies from circular bioeconomy perspectives. Bioresour Technol 351:127038. https://doi.org/10.1016/j.biortech.2022.127038
Zhang Y, Wu H, Yuan C et al (2019) Growth, biochemical composition, and photosynthetic performance of Scenedesmus acuminatus during nitrogen starvation and resupply. J Appl Phycol 31:2797–2809. https://doi.org/10.1007/s10811-019-01783-z
Zarrinmehr MJ, Farhadian O, Heyrati FP, Keramat J, Koutra E, Kornaros M, Daneshvar E (2020) Effect of nitrogen concentration on the growth rate and biochemical composition of the microalga, Isochrysis galbana. Egypt J Aquat Res 46:153–158. https://doi.org/10.1016/j.ejar.2019.11.003
Zhu Y, Zhao X, Zhang X, Liu H (2019) Extraction, structural and functional properties of Haematococcus pluvialis protein after pigment removal. Int J Biol Macromol 140:1073–1083. https://doi.org/10.1016/j.ijbiomac.2019.08.209
Minhas AK, Hodgson P, Barrow CJ, Adholeya A (2016) A Review on the assessment of stress conditions for simultaneous production of microalgal lipids and carotenoids. Front Microbiol 7:546. https://doi.org/10.3389/fmicb.2016.00546
Kim DY, Vijayan D, Praveenkumar R, Han J-I, Lee K, Park J-Y, Chang W-S, Lee J-S, Oh Y-K (2016) Cell-wall disruption and lipid/astaxanthin extraction from microalgae: Chlorella and Haematococcus. Bioresour Technol 199:300–310. https://doi.org/10.1016/j.biortech.2015.08.107
Yu C, Li X, Han B, Zhao Y, Geng S, Ning D, Ma T, Yu X (2021) Simultaneous improvement of astaxanthin and lipid production of Haematococcus pluvialis by using walnut shell extracts. Algal Res 54:102171. https://doi.org/10.1016/j.algal.2020.102171
Nishshanka GKSH, Liyanaarachchi VC, Nimarshana PHV, Ariyadasa TU, Chang JS (2022) Haematococcus pluvialis: A potential feedstock for multiple-product biorefining. J Clean Prod 344:131103. https://doi.org/10.1016/j.jclepro.2022.131103
Behera B, Acharya A, Gargey IA, Aly N, Balasubramanian P (2019) Bioprocess engineering principles of microalgal cultivation for sustainable biofuel production. Bioresour Technol Reports 5:297–316. https://doi.org/10.1016/j.biteb.2018.08.001
Subhash GV, Rajvanshi M, Kumar GR, Sagaram US, Prasad V, Govindachary S, Dasgupta S (2022) Challenges in microalgal biofuel production: A perspective on techno economic feasibility under biorefinery stratagem. Bioresour Technol 343:126155. https://doi.org/10.1016/j.biortech.2021.126155
Siddiki SYA, Mofijur M, Kumar PS, Ahmed SF, Inayat A, Kusumo F, Badruddin IA, Khan TY, Nghiem LD, Ong HC, Mahlia TM (2022) Microalgae biomass as a sustainable source for biofuel, biochemical and biobased value-added products: an integrated biorefinery concept. Fuel 307:121782. https://doi.org/10.1016/j.fuel.2021.121782
Banu JR, Preethi KS, Kumar G (2020) Microalgae based biorefinery promoting circular bioeconomy-techno economic and life-cycle analysis. Bioresour Technol 302:122822. https://doi.org/10.1016/j.biortech.2020.122822
Thawechai T, Cheirsilp B, Louhasakul Y, Boonsawang P, Prasertsan P (2016) Mitigation of carbon dioxide by oleaginous microalgae for lipids and pigments production: effect of light illumination and carbon dioxide feeding strategies. Bioresour Technol 219:139–149. https://doi.org/10.1016/j.biortech.2016.07.109
Wang F, Gao B, Wu M, Huang L, Zhang C (2019) A novel strategy for the hyper-production of astaxanthin from the newly isolated microalga Haematococcus pluvialis JNU35. Algal Res 39:101466. https://doi.org/10.1016/j.algal.2019.101466
Nahidian B, Ghanati F, Shahbazi M, Soltani N (2018) Effect of nutrients on the growth and physiological features of newly isolated Haematococcus pluvialis TMU1. Bioresour Technol 255:229–237. https://doi.org/10.1016/j.biortech.2018.01.130
Fu L, Li Q, Yan G, Zhou D, Crittenden JC (2019) Hormesis effects of phosphorus on the viability of Chlorella regularis cells under nitrogen limitation. Biotechnol Biofuels 12:121. https://doi.org/10.1186/s13068-019-1458-z
Pancha I, Chokshi K, George B, Ghosh T, Paliwal C, Maurya R, Mishra S (2014) Nitrogen stress triggered biochemical and morphological changes in the microalgae Scenedesmus sp. CCNM 1077. Bioresour Technol 156:146–154. https://doi.org/10.1016/j.biortech.2014.01.025
Zhang WW, Zhou XF, Zhang YL, Cheng PF, Ma R, Cheng WL, Chu HQ (2018) Enhancing astaxanthin accumulation in Haematococcus pluvialis by coupled light intensity and nitrogen starvation in column photobioreactors. J Microbiol Biotechnol 28:2019–2028. https://doi.org/10.4014/jmb.1807.07008
Moheimani NR, Borowitzka MA, Isdepsky A, Sing SF (2013) Standard methods for measuring growth of algae and their composition. In: Algae for biofuels and energy. Springer Netherlands, Dordrecht, pp 265–284. https://doi.org/10.1007/978-94-007-5479-9_16
Lichtenthaler HK, Buschmann C (2001) Chlorophylls and carotenoids: measurement and characterization by UV-VIS spectroscopy. Curr Protoc Food Anal Chem 1:F4.3.1-F4.3.8. https://doi.org/10.1002/0471142913.faf0403s01
Liyanaarachchi VC, Nishshanka GKSH, Premaratne RGMM et al (2020) Astaxanthin accumulation in the green microalga Haematococcus pluvialis: effect of initial phosphate concentration and stepwise/continuous light stress. Biotechnol Reports 28:e00538. https://doi.org/10.1016/j.btre.2020.e00538
Klin M, Pniewski F, Latała A (2018) Characteristics of the growth rate and lipid production in fourteen strains of Baltic green microalgae. Oceanol Hydrobiol Stud 47:10–18. https://doi.org/10.1515/ohs-2018-0002
DuBois M, Gilles KA, Hamilton JK, Rebers PA, Smith F (1956) Colorimetric method for determination of sugars and related substances. Anal Chem 28:350–356. https://doi.org/10.1021/ac60111a017
Kobayashi N, Noel EA, Barnes A, Watson A, Rosenberg JN, Erickson G, Oyler GA (2013) Characterization of three Chlorella sorokiniana strains in anaerobic digested effluent from cattle manure. Bioresour Technol 150:377–386. https://doi.org/10.1016/j.biortech.2013.10.032
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254. https://doi.org/10.1006/abio.1976.9999
Breuer G, Evers WAC, de Vree JH, Kleinegris DMM, Martens DE, Wijffels RH, Lamers PP (2013) Analysis of fatty acid content and composition in microalgae. J Vis Exp 50628. https://doi.org/10.3791/50628
Song M, Pei H, Hu W, Ma G (2013) Evaluation of the potential of 10 microalgal strains for biodiesel production. Bioresour Technol 141:245–251. https://doi.org/10.1016/j.biortech.2013.02.024
Arguelles ED, Laurena AC, Monsalud RG, Martinez-Goss MR (2018) Fatty acid profile and fuel-derived physico-chemical properties of biodiesel obtained from an indigenous green microalga, Desmodesmus sp. (I-AU1), as potential source of renewable lipid and high quality biodiesel. J Appl Phycol 30:411–419. https://doi.org/10.1007/s10811-017-1264-6
Nautiyal P, Subramanian KA, Dastidar MG (2014) Production and characterization of biodiesel from algae. Fuel Process Technol 120:79–88. https://doi.org/10.1016/j.fuproc.2013.12.003
Ördög V, Stirk WA, Bálint P et al (2012) Changes in lipid, protein and pigment concentrations in nitrogen-stressed Chlorella minutissima cultures. J Appl Phycol 24:907–914. https://doi.org/10.1007/s10811-011-9711-2
Tarazona Delgado R, dos Guarieiro M, S, Antunes PW, et al (2021) Effect of nitrogen limitation on growth, biochemical composition, and cell ultrastructure of the microalga Picocystis salinarum. J Appl Phycol 33:2083–2092. https://doi.org/10.1007/s10811-021-02462-8
Dean AP, Sigee DC, Estrada B, Pittman JK (2010) Using FTIR spectroscopy for rapid determination of lipid accumulation in response to nitrogen limitation in freshwater microalgae. Bioresour Technol 101:4499–4507. https://doi.org/10.1016/j.biortech.2010.01.065
Liu T, Chen Z, Xiao Y, Yuan M, Zhou C, Liu G, Fang J, Yang B (2022) Biochemical and morphological changes triggered by nitrogen stress in the oleaginous microalga Chlorella vulgaris. Microorganisms 10:566. https://doi.org/10.3390/microorganisms10030566
Zhang Y-M, Chen H, He C-L, Wang Q (2013) Nitrogen starvation induced oxidative stress in an oil-producing green alga Chlorella sorokiniana C3. PLoS ONE 8:e69225. https://doi.org/10.1371/journal.pone.0069225
Ma X, Liu J, Liu B et al (2016) Physiological and biochemical changes reveal stress-associated photosynthetic carbon partitioning into triacylglycerol in the oleaginous marine alga Nannochloropsis oculata. Algal Res 16:28–35. https://doi.org/10.1016/j.algal.2016.03.005
Young EB, Beardall J (2003) Photosynthetic function in Dunaliella tertiolecta (Chlorophyta) during a nitrogen starvation and recovery cycle. J Phycol 39:897–905. https://doi.org/10.1046/j.1529-8817.2003.03042.x
Li F, Cai M, Wu Y, Lian Q, Qian Z, Luo J, Zhang Y, Zhang N, Li C, Huang X (2022) Effects of nitrogen and light intensity on the astaxanthin accumulation in motile cells of Haematococcus pluvialis. Front Mar Sci 9:. https://doi.org/10.3389/fmars.2022.909237
Niizawa I, Espinaco BY, Leonardi JR, Heinrich JM, Sihufe AG (2018) Enhancement of astaxanthin production from Haematococcus pluvialis under autotrophic growth conditions by a sequential stress strategy. Prep Biochem Biotechnol 48:528–534. https://doi.org/10.1080/10826068.2018.1466159
Hosseini A, Jazini M, Mahdieh M, Karimi K (2020) Efficient superantioxidant and biofuel production from microalga Haematococcus pluvialis via a biorefinery approach. Bioresour Technol 306:123100. https://doi.org/10.1016/j.biortech.2020.123100
Li T, Xu J, Gao B, Xiang W, Li A, Zhang C (2016) Morphology, growth, biochemical composition and photosynthetic performance of Chlorella vulgaris (Trebouxiophyceae) under low and high nitrogen supplies. Algal Res 16:481–491. https://doi.org/10.1016/j.algal.2016.04.008
Courchesne NMD, Parisien A, Wang B, Lan CQ (2009) Enhancement of lipid production using biochemical, genetic and transcription factor engineering approaches. J Biotechnol 141:31–41. https://doi.org/10.1016/j.jbiotec.2009.02.018
Pal D, Khozin-Goldberg I, Cohen Z, Boussiba S (2011) The effect of light, salinity, and nitrogen availability on lipid production by Nannochloropsis sp. Appl Microbiol Biotechnol 90:1429–1441. https://doi.org/10.1007/s00253-011-3170-1
Behera B, Selvam SM, Dey B, Balasubramanian P (2020) Algal biodiesel production with engineered biochar as a heterogeneous solid acid catalyst. Bioresour Technol 310:123392. https://doi.org/10.1016/j.biortech.2020.123392
Chen J, Li J, Dong W, Zhang X, Tyagi RD, Drogui P, Surampalli RY (2018) The potential of microalgae in biodiesel production. Renew Sustain Energy Rev 90:336–346. https://doi.org/10.1016/j.rser.2018.03.073
Alptekin E, Canakci M (2008) Determination of the density and the viscosities of biodiesel-diesel fuel blends. Renew Energy 33:2623–2630. https://doi.org/10.1016/j.renene.2008.02.020
Hoekman SK, Broch A, Robbins C, Ceniceros E, Natarajan M (2012) Review of biodiesel composition, properties, and specifications. Renew Sustain Energy Rev 16:143–169. https://doi.org/10.1016/j.rser.2011.07.143
Peterson CL, Taberski JS, Thompson JC, Chase CL (2000) The effect of biodiesel feedstock on regulated emmisions in chassis dynamometer tests of a pickup truck. Trans ASAE 43:1371–1381. https://doi.org/10.13031/2013.3034
Funding
This work was supported by Shiraz University Research Council under grant number 256248.
Author information
Authors and Affiliations
Contributions
Zahra Zarei: investigation, writing (original draft), methodology, formal analysis. Hajar Zamani: formal analysis, conceptualization, supervision, writing (review and editing), funding acquisition.
Corresponding author
Ethics declarations
Conflict of Interest statement
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Zarei, Z., Zamani, H. Biorefinery Potential of Microalga Haematococcus pluvialis to Produce Astaxanthin and Biodiesel Under Nitrogen Deprivation. Bioenerg. Res. 16, 1777–1788 (2023). https://doi.org/10.1007/s12155-022-10554-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12155-022-10554-7