Abstract
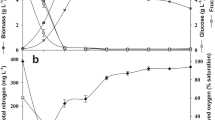
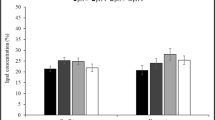
In this study, lipid accumulation and biodiesel production potentials of novel yeast isolates grown in pumpkin peel were investigated. Effect of some critical parameters on lipid production such as initial biomass loading (5–20%), type and concentration of nitrogen source (cheese whey and (NH4)2SO4, 0.25–2 gL−1), and incubation time (0–96 h) were also determined. Furthermore, the effect of biomass loading (0.5–2 gL−1) and catalyst concentration (NaOH 1–3%) with methanol on fatty acid methyl ester (FAME) yields were optimized with response surface methodology (RSM). One yeast isolate showed the highest lipid production, and this strain was identified as Candida boidinii. The maximum lipid accumulation of the novel isolate was observed as 45.6% in the presence of 10% initial pumpkin peel loading, 0.5 gL−1 cheese whey, and 72-h incubation time. The highest FAME (C16-C18) yield was 92.3% when 2 gL−1 biomass and 3% catalyst (NaOH) were used. These results showed that Candida boidinii is a promising microorganism for biodiesel production and pumpkin peel supports the microbial growth.





Similar content being viewed by others
References
Tabatabaei M, Aghbashlo M (2020) The critical role of advanced sustainability assessment tools in enhancing the real-world application of biofuels. Acta Innov 37:67–73. https://doi.org/10.32933/ActaInnovations.37.6
Cherubini F (2010) The biorefinery concept: using biomass instead of oil for producing energy and chemicals. Energy Convers Manag 51:1412–1421. https://doi.org/10.1016/j.enconman.2010.01.015
Kakkad H, Khot M, Zinjarde S, RaviKumar A (2015) Biodiesel production by direct in situ transesterification of an oleaginous tropical mangrove fungus grown on untreated agro-residues and evaluation of its fuel properties. Bioenergy Res 8:1788–1799. https://doi.org/10.1007/s12155-015-9626-x
Mandari V, Devarai SK (2021) Biodiesel production using homogeneous, heterogeneous, and enzyme catalysts via transesterification and esterification reactions: a critical review. Bioenergy Res. https://doi.org/10.1007/s12155-021-10333-w
Festel G, Würmseher M, Rammer C (2014) Scaling and learning effects of biofuels conversion technologies. Energy Technol 2:612–617. https://doi.org/10.1002/ente.201400014
Morais ARC, Da Costa Lopes AM, Costa P et al (2014) Cattle fat valorisation through biofuel production by hydrogenation in supercritical carbon dioxide. RSC Adv 4:32081–32091. https://doi.org/10.1039/c4ra05225k
Singh D, Sharma D, Soni SL et al (2020) A review on feedstocks, production processes, and yield for different generations of biodiesel. Fuel 262:116553. https://doi.org/10.1016/j.fuel.2019.116553
Koreti D, Kosre A, Jadhav SK, Chandrawanshi NK (2022) A comprehensive review on oleaginous bacteria: an alternative source for biodiesel production. Bioresour Bioprocess 9:1–19. https://doi.org/10.1186/s40643-022-00527-1
Escorsim AM, Cordeiro CS, Ramos LP et al (2015) Assessment of biodiesel purification using CO2 at high pressures. J Supercrit Fluids 96:68–76. https://doi.org/10.1016/j.supflu.2014.08.013
Bhuiya MMK, Rasul MG, Khan MMK et al (2016) Prospects of 2nd generation biodiesel as a sustainable fuel - Part: 1 selection of feedstocks, oil extraction techniques and conversion technologies. Renew Sustain Energy Rev 55:1109–1128. https://doi.org/10.1016/j.rser.2015.04.163
Leiva-Candia DE, Pinzi S, Redel-Macías MD et al (2014) The potential for agro-industrial waste utilization using oleaginous yeast for the production of biodiesel. Fuel 123:33–42. https://doi.org/10.1016/j.fuel.2014.01.054
Bhatia SK, Gurav R, Choi TR et al (2019) A clean and green approach for odd chain fatty acids production in Rhodococcus sp. YHY01 by medium engineering. Bioresour Technol 286:121383. https://doi.org/10.1016/j.biortech.2019.121383
Chebbi H, Leiva-Candia D, Carmona-Cabello M et al (2019) Biodiesel production from microbial oil provided by oleaginous yeasts from olive oil mill wastewater growing on industrial glycerol. Ind Crops Prod 139:111535. https://doi.org/10.1016/j.indcrop.2019.111535
Chopra J, Tiwari BR, Dubey BK, Sen R (2020) Environmental impact analysis of oleaginous yeast based biodiesel and bio-crude production by life cycle assessment. J Clean Prod 271:122349. https://doi.org/10.1016/j.jclepro.2020.122349
Fakas S, Papanikolaou S, Galiotou-Panayotou M et al (2008) Organic nitrogen of tomato waste hydrolysate enhances glucose uptake and lipid accumulation in Cunninghamella echinulata. J Appl Microbiol 105:1062–1070. https://doi.org/10.1111/j.1365-2672.2008.03839.x
Intasit R, Cheirsilp B, Louhasakul Y et al (2020) Valorization of palm biomass wastes for biodiesel feedstock and clean solid biofuel through non-sterile repeated solid-state fermentation. Bioresour Technol 298:122551. https://doi.org/10.1016/j.biortech.2019.122551
Sathiyamoorthi E, Dikshit PK, Kumar P, Kim BS (2020) Co-fermentation of agricultural and industrial waste by Naganishia albida for microbial lipid production in fed-batch fermentation. J Chem Technol Biotechnol 95:813–821. https://doi.org/10.1002/jctb.6271
Schieber A, Stintzing FC, Carle R (2001) By-products of plant food processing as a source of valuable compounds-recent developments. Trends Food Sci Technol 12:401–413. https://doi.org/10.1016/b978-0-08-100596-5.21346-2
Yang R, Meng D, Hu X et al (2013) Saccharification of pumpkin residues by coculturing of trichoderma reesei RUT-C30 and phanerochaete chrysosporium burdsall with delayed inoculation timing. J Agric Food Chem 61:9192–9199. https://doi.org/10.1021/jf402199j
Fissore EN, Ponce NM, Stortz CA et al (2007) Characterisation of fiber obtained from pumpkin (cucumis moschata duch.) mesocarp through enzymatic treatment. Food Sci Technol Int 13:141–151. https://doi.org/10.1177/1082013207077914
Santana NB, Teixeira Dias JC, Rezende RP et al (2018) Production of xylitol and bio-detoxification of cocoa pod husk hemicellulose hydrolysate by Candida boidinii XM02G. PLoS ONE 13:1–15. https://doi.org/10.1371/journal.pone.0195206
Gonçalves DB, Batista AF, Rodrigues MQRB et al (2013) Ethanol production from macaúba (Acrocomia aculeata) presscake hemicellulosic hydrolysate by Candida boidinii UFMG14. Bioresour Technol 146:261–266. https://doi.org/10.1016/j.biortech.2013.07.075
Papanikolaou S, Rontou M, Belka A et al (2017) Conversion of biodiesel-derived glycerol into biotechnological products of industrial significance by yeast and fungal strains. Eng Life Sci 17:262–281. https://doi.org/10.1002/elsc.201500191
Glass NL, Donaldson GC (1995) Development of primer sets designed for use with the PCR to amplify conserved genes from filamentous ascomycetes. Appl Environ Microbiol 61:1323–1330. https://doi.org/10.1128/aem.61.4.1323-1330.1995
Li Y, Zhao Z (Kent), Bai F (2007) High-density cultivation of oleaginous yeast Rhodosporidium toruloides Y4 in fed-batch culture. Enzyme Microb Technol 41:312–317. https://doi.org/10.1016/j.enzmictec.2007.02.008
Miller GL (1959) Use of dinitrosalicylic acid reagent for determination of reducing sugar. Anal Chem 31:426–428. https://doi.org/10.1021/ac60147a030
Yang RH, Su JH, Shang JJ et al (2018) Evaluation of the ribosomal DNA internal transcribed spacer (ITS), specifically ITS1 and ITS2, for the analysis of fungal diversity by deep sequencing. PLoS ONE 13:1–17. https://doi.org/10.1371/journal.pone.0206428
Schulze I, Hansen S, Großhans S et al (2014) Characterization of newly isolated oleaginous yeasts - Cryptococcus podzolicus, Trichosporon porosum and Pichia segobiensis. AMB Express 4:1–11. https://doi.org/10.1186/s13568-014-0024-0
Liu L, Chen J, Lim PE, Wei D (2018) Dual-species cultivation of microalgae and yeast for enhanced biomass and microbial lipid production. J Appl Phycol 30:2997–3007. https://doi.org/10.1007/s10811-018-1526-y
Jeevan Kumar SP, Banerjee R (2019) Enhanced lipid extraction from oleaginous yeast biomass using ultrasound assisted extraction: a greener and scalable process. Ultrason Sonochem 52:25–32. https://doi.org/10.1016/j.ultsonch.2018.08.003
Koppram R, Tomás-Pejó E, Xiros C, Olsson L (2014) Lignocellulosic ethanol production at high-gravity: challenges and perspectives. Trends Biotechnol 32:46–53. https://doi.org/10.1016/j.tibtech.2013.10.003
Thangavelu K, Sundararaju P, Srinivasan N et al (2020) Simultaneous lipid production for biodiesel feedstock and decontamination of sago processing wastewater using Candida tropicalis ASY2. Biotechnol Biofuels 13:1–14. https://doi.org/10.1186/s13068-020-01676-1
Kitcha S, Cheirsilp B (2011) Screening of oleaginous yeasts and optimization for lipid production using crude glycerol as a carbon source. Energy Procedia 9:274–282. https://doi.org/10.1016/j.egypro.2011.09.029
Poontawee R, Yongmanitchai W, Limtong S (2018) Lipid production from a mixture of sugarcane top hydrolysate and biodiesel-derived crude glycerol by the oleaginous red yeast, Rhodosporidiobolus fluvialis. Process Biochem 66:150–161. https://doi.org/10.1016/j.procbio.2017.11.020
Gohel HR, Ghosh SK, Braganza VJ (2013) Yeast as a viable and prolonged feedstock for biodiesel production. Int J Renew Energy Res 3:126–131. https://doi.org/10.20508/ijrer.93684
Hossain E, Sultana SA, Karim MH, Ahmed I (2015) Vegetable peels: a promising feed resource for livestock. J Anim Feed Res 5:33–39
Mala SK, Kurian AE (2016) Nutritional Composition and Antioxidant Activity of Pumpkin Wastes. Int J Pharm Chem Biol Sci 6:336–344
Gong Z, Shen H, Wang Q et al (2013) Efficient conversion of biomass into lipids by using the simultaneous saccharification and enhanced lipid production process. Biotechnol Biofuels 6.https://doi.org/10.1186/1754-6834-6-36
Nababan MYS, Fatriasari W, Wistara NJ (2020) Response surface methodology for enzymatic hydrolysis optimization of jabon alkaline pulp with Tween 80 surfactant addition. Biomass Convers Biorefinery. https://doi.org/10.1007/s13399-020-00807-w
Yellapu SK, Bezawada J, Kaur R et al (2016) Detergent assisted lipid extraction from wet yeast biomass for biodiesel: a response surface methodology approach. Bioresour Technol 218:667–673. https://doi.org/10.1016/j.biortech.2016.07.011
Selvakumar P, Sivashanmugam P (2019) Ultrasound assisted oleaginous yeast lipid extraction and garbage lipase catalyzed transesterification for enhanced biodiesel production. Energy Convers Manag 179:141–151. https://doi.org/10.1016/j.enconman.2018.10.051
Tacias-Pascacio VG, Torrestiana-Sánchez B, Dal Magro L et al (2019) Comparison of acid, basic and enzymatic catalysis on the production of biodiesel after RSM optimization. Renew Energy 135:1–9. https://doi.org/10.1016/j.renene.2018.11.107
Verma P, Sharma MP, Dwivedi G (2016) Prospects of bio-based alcohols for Karanja biodiesel production: an optimisation study by response surface methodology. Fuel 183:185–194. https://doi.org/10.1016/j.fuel.2016.06.062
Ma F, Clements LD, Hanna MA (1998) The effects of catalyst, free fatty acids, and water on transesterification of beef tallow. Trans Am Soc Agric Eng 41:1261–1264. https://doi.org/10.13031/2013.17292
Thliveros P, Uçkun Kiran E, Webb C (2014) Microbial biodiesel production by direct methanolysis of oleaginous biomass. Bioresour Technol 157:181–187. https://doi.org/10.1016/j.biortech.2014.01.111
Pullen J, Saeed K (2015) Investigation of the factors affecting the progress of base-catalyzed transesterification of rapeseed oil to biodiesel FAME. Fuel Process Technol 130:127–135. https://doi.org/10.1016/j.fuproc.2014.09.013
Siwina S, Leesing R (2021) Bioconversion of durian (Durio zibethinus Murr.) peel hydrolysate into biodiesel by newly isolated oleaginous yeast Rhodotorula mucilaginosa KKUSY14. Renew Energy 163:237–245. https://doi.org/10.1016/j.renene.2020.08.138
Miao Z, Tian X, Liang W et al (2020) Bioconversion of corncob hydrolysate into microbial lipid by an oleaginous yeast Rhodotorula taiwanensis AM2352 for biodiesel production. Renew Energy 161:91–97. https://doi.org/10.1016/j.renene.2020.07.007
Kuan IC, Kao WC, Chen CL, Yu CY (2018) Microbial biodiesel production by direct transesterification of rhodotorula glutinis biomass. Energies 11. https://doi.org/10.3390/en11051036
Leung DYC, Guo Y (2006) Transesterification of neat and used frying oil: optimization for biodiesel production. Fuel Process Technol 87:883–890. https://doi.org/10.1016/j.fuproc.2006.06.003
Eevera T, Rajendran K, Saradha S (2009) Biodiesel production process optimization and characterization to assess the suitability of the product for varied environmental conditions. Renew Energy 34:762–765. https://doi.org/10.1016/j.renene.2008.04.006
Katre G, Raskar S, Zinjarde S et al (2018) Optimization of the in situ transesterification step for biodiesel production using biomass of Yarrowia lipolytica NCIM 3589 grown on waste cooking oil. Energy 142:944–952. https://doi.org/10.1016/j.energy.2017.10.082
Talebi AF, Tabatabaei M, Chisti Y (2014) BiodieselAnalyzer: a user-friendly software for predicting the properties of prospective biodiesel. Biofuel Res J 2:55–57. https://doi.org/10.18331/brj2015.1.2.4
Rozina AM, Zafar M et al (2017) Biodiesel synthesis from Saussurea heteromalla (D.Don) Hand-Mazz integrating ethanol production using biorefinery approach. Energy 141:1810–1818. https://doi.org/10.1016/j.energy.2017.11.074
Anwar F, Rashid U, Ashraf M, Nadeem M (2010) Okra (Hibiscus esculentus) seed oil for biodiesel production. Appl Energy 87:779–785. https://doi.org/10.1016/j.apenergy.2009.09.020
Funding
This study was supported by Ankara University Research Foundation. Project Number: 20L0430004.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing Interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Demiray, E., Karatay, S.E. & Dönmez, G. Optimization Study for Enhanced Biodiesel Production by Novel Yeast Isolates Cultivated in Dilute Acid Pretreated Pumpkin Peel. Bioenerg. Res. 15, 1472–1481 (2022). https://doi.org/10.1007/s12155-022-10483-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12155-022-10483-5