Abstract
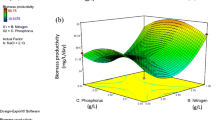
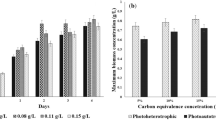
The initial biomass density had a significant effect on the growth behaviour of Leptolyngbya foveolarum HNBGU-001 in the batch culture. The test organism produced substantial amounts of biomass and lipids when allowed to grow at 40 °C and pH 8.0. The Placket-Burman design appropriately indicated the positive and negative influences of the tested nutrient factors on biomass and lipid productivities of L. foveolarum. Employing the Box-Behnken design of response surface methodology, the elevated concentrations of sulphate-S (300 mg/L) and carbonate-C (45.39 mg/L) but relatively low levels of phosphate-P (10 mg/L) and nitrate-N (375 mg/L) were identified as the optimal nutritional requirements of the test organism for the maximum lipid productivity (49.60 ± 0.70 mg/L/day) along with high lipid content (32.10 ± 0.40% dry cell weight) and biomass productivity (154.80 ± 3.60 mg/L/day). These maximized responses of biomass productivity, lipid content, and lipid productivity were respectively 2.8-, 2.4-, and 6.8-fold higher than their corresponding values under the unoptimized conditions. FAME analysis of lipids obtained from the test organism revealed some promising features for their use as algal biodiesel feedstock. Nonetheless, the test organism seems to have better potential than several other microalgae for use in biofuel production as it can give a high output of biomass as also lipids at elevated temperature prevalent in outdoor conditions of subtropical and tropical regions.





Similar content being viewed by others
Data Availability
All the data are available with the corresponding author and may be provided on request.
References
Singh P, Singh RK, Kumar D (2018) Microalgae: potential agents for carbon dioxide mitigation. In: Kashyap PL, Srivastava AK, Tiwari SP, Kumar S (eds) Microbes for climate resilient agriculture, first. John Wiley & Sons, Inc., Hoboken, pp 57–74. https://doi.org/10.1002/9781119276050.ch4
Shanmugam S, Mathimani T, Anto S, Sudhakar MP, Kumar SS, Pugazhendhi A (2020) Cell density, lipidomic profile, and fatty acid characterization as selection criteria in bioprospecting of microalgae and cyanobacterium for biodiesel production. Bioresour Technol 304:123061. https://doi.org/10.1016/j.biortech.2020.123061
DOE (United States Department of Energy) (2016) National algal biofuels technology review. https://www.energy.gov/sites/prod/files/2016/06/f33/national_algal_biofuels_technology_review.pdf. Accessed 24 June 2017
Veillette M, Giroir-Fendler A, Faucheux N, Heitz M (2018) Biodiesel from microalgae lipids: from inorganic carbon to energy production. Biofuels 9:175–202. https://doi.org/10.1080/17597269.2017.1289667
Fields MW, Hise A, Lohman EJ, Bell T, Gardner RD, Corredor L, Moll K, Peyton BM, Characklis GW, Gerlach R (2014) Sources and resources: importance of nutrients, resource allocation, and ecology in microalgal cultivation for lipid accumulation. Appl Microbiol Biotechnol 98:4805–4816. https://doi.org/10.1007/s00253-014-5694-7
Kim JH, Choi W, Jeon SM, Kim T, Park A, Kim J, Heo SJ, Oh C, Shim WB, Kang DH (2015) Isolation and characterization of Leptolyngbya sp. KIOST-1, a basophilic and euryhaline filamentous cyanobacterium from an open paddle-wheel raceway Arthrospira culture pond in Korea. J Appl Microbiol 119:1597–1612. https://doi.org/10.1111/jam.12961
Yang L, Chen J, Qin S, Zeng M, Jiang Y, Hu L, Xiao P, Hao W, Hu Z, Lei A, Wang J (2018) Growth and lipid accumulation by different nutrients in the microalga Chlamydomonas reinhardtii. Biotechnol Biofuels 11:1–12. https://doi.org/10.1186/s13068-018-1041-z
Sharma D, Yadav KD, Kumar S (2018) Biotransformation of flower waste composting: optimization of waste combinations using response surface methodology. Bioresour Technol 270:198–207. https://doi.org/10.1016/j.biortech.2018.09.036
Sompong U, Hawkins PR, Besley C, Peerapornpisal Y (2005) The distribution of cyanobacteria across physical and chemical gradients in hot springs in northern Thailand. FEMS Microbiol Ecol 52:365–376. https://doi.org/10.1016/j.femsec.2004.12.007
Santhakumaran P, Kookal SK, Mathew L, Ray JG (2019) Bioprospecting of three rapid-growing freshwater green algae, promising biomass for biodiesel production. Bioenergy Res 12:680–693. https://doi.org/10.1007/s12155-019-09990-9
Thangavel K, Radha Krishnan P, Nagaiah S, Kuppusamy S, Chinnasamy S, Rajadorai JS, Nellaiappan Olaganathan G, Dananjeyan B (2018) Growth and metabolic characteristics of oleaginous microalgal isolates from Nilgiri biosphere reserve of India. BMC Microbiol 18:1–17. https://doi.org/10.1186/s12866-017-1144-x
Smith-Bädorf HD, Chuck CJ, Mokebo KR, MacDonald H, Davidson MG, Scott RJ (2013) Bioprospecting the thermal waters of the Roman baths: isolation of oleaginous species and analysis of the FAME profile for biodiesel production. AMB Express 3:1–14. https://doi.org/10.1186/2191-0855-3-9
Patel VK, Sundaram S, Patel AK, Kalra A (2018) Characterization of seven species of cyanobacteria for high-quality biomass production. Arab J Sci Eng 43:109–121. https://doi.org/10.1007/s13369-017-2666-0
Singh J, Thakur IS (2015) Evaluation of cyanobacterial endolith Leptolyngbya sp. ISTCY101, for integrated wastewater treatment and biodiesel production: a toxicological perspective. Algal Res 11:294–303. https://doi.org/10.1016/j.algal.2015.07.010
Hughes AR, Sulesky A, Andersson B, Peers G (2018) Sulfate amendment improves the growth and bioremediation capacity of a cyanobacteria cultured on municipal wastewater centrate. Algal Res 32:30–37. https://doi.org/10.1016/j.algal.2018.03.004
Tao R, Bair R, Lakaniemi AM, van Hullebusch ED, Rintala JA (2019) Use of factorial experimental design to study the effects of iron and sulfur on growth of Scenedesmus acuminatus with different nitrogen sources. J Appl Phycol 32:221–231. https://doi.org/10.1007/s10811-019-01915-5
Sakarika M, Kornaros M (2016) Effect of pH on growth and lipid accumulation kinetics of the microalga Chlorella vulgaris grown heterotrophically under sulfur limitation. Bioresour Technol 219:694–701. https://doi.org/10.1016/j.biortech.2016.08.033
Ernst A, Deicher M, Herman PMJ, Wollenzien UIA (2005) Nitrate and phosphate affect cultivability of cyanobacteria from environments with low nutrient levels. Appl Environ Microbiol 71:3379–3383. https://doi.org/10.1128/AEM.71.6.3379-3383.2005
Huang B, Marchand J, Blanckaert V, Lukomska E, Ulmann L, Wielgosz-Collin G, Rabesaotra V, Moreau B, Bougaran G, Mimouni V, Morant-Manceau A (2019) Nitrogen and phosphorus limitations induce carbon partitioning and membrane lipid remodelling in the marine diatom Phaeodactylum tricornutum. Eur J Phycol 54:342–358. https://doi.org/10.1080/09670262.2019.1567823
Daniel RM, Cowan DA (2000) Biomolecular stability and life at high temperatures. Cell Mol Life Sci 57:250–264. https://doi.org/10.1007/PL00000688
Patel A, Matsakas L, Rova U, Christakopoulos P (2019) A perspective on biotechnological applications of thermophilic microalgae and cyanobacteria. Bioresour Technol 278:424–434. https://doi.org/10.1016/j.biortech.2019.01.063
Maslova IP, Mouradyan EA, Lapina SS, Klyachko-Gurvich GL, Los DA (2004) Lipid fatty acid composition and thermophilicity of cyanobacteria. Russ J Plant Physiol 51:353–360. https://doi.org/10.1023/B:RUPP.0000028681.40671.8d
Qv XY, Guo YY, Jiang JG (2014) Assessment of the effects of nutrients on biomass and lipid accumulation in Dunaliella tertiolecta using a response surface methodology. RSC Adv 4:42202–42210. https://doi.org/10.1039/c4ra04192e
Suthar S, Verma R, Kumar K (2018) Production of Chlorella vulgaris under varying nutrient and abiotic conditions: a potential microalga for bioenergy feedstock. Process Saf Environ Prot 113:141–148. https://doi.org/10.1016/j.psep.2017.09.018
Mera R, Torres E, Abalde J (2016) Effects of sodium sulfate on the freshwater microalga Chlamydomonas moewusii: implications for the optimization of algal culture media. J Phycol 52:75–88. https://doi.org/10.1111/jpy.12367
Kanaga K, Pandey A, Kumar S, Geetanjali (2016) Multi-objective optimization of media nutrients for enhanced production of algae biomass and fatty acid biosynthesis from Chlorella pyrenoidosa NCIM 2738. Bioresour Technol 200:940–950. https://doi.org/10.1016/j.biortech.2015.11.017
Pandey A, Srivastava S, Kumar S (2019) Sequential optimization of essential nutrients addition in simulated dairy effluent for improved Scenedesmus sp ASK22 growth, lipid production and nutrients removal. Biomass Bioenergy 128:105319. https://doi.org/10.1016/j.biombioe.2019.105319
Chakravarty S, Mallick N (2019) Optimization of lipid accumulation in an aboriginal green microalga Selenastrum sp. GA66 for biodiesel production. Biomass Bioenergy 126:1–13. https://doi.org/10.1016/j.biombioe.2019.05.006
Rippka R, Deruelles J, Waterbury JB (1979) Generic assignments, strain histories and properties of pure cultures of cyanobacteria. J Gen Microbiol 111:1–61. https://doi.org/10.1099/00221287-111-1-1
Thomas J, Jayachithra EV (2015) Growth kinetics of Chlorococcum humicola - a potential feedstock for biomass with biofuel properties. Ecotoxicol Environ Saf 121:258–262. https://doi.org/10.1016/j.ecoenv.2015.03.008
Rakić T, Jančić-Stojanovic B, Malenović A et al (2012) Demasking large dummy effects approach in revealing important interactions in Plackett-Burman experimental design. J Chemom 26:518–525. https://doi.org/10.1002/cem.2461
Plackett RL, Burman JP (1946) The design of optimum multifactorial experiments. Biometrika 33:305–325
Wang S, Cao M, Wang B, Deng R, Gao Y, Liu P (2019) Optimization of growth requirements and scale-up cultivation of freshwater algae Desmodesmus armatus using response surface methodology. Aquac Res 50:3313–3325. https://doi.org/10.1111/are.14290
Markou G, Vandamme D, Muylaert K (2014) Microalgal and cyanobacterial cultivation: the supply of nutrients. Water Res 65:186–202. https://doi.org/10.1016/j.watres.2014.07.025
Bligh EG, Dyer WJ (1959) A rapid method of total lipid extraction and purification. Can J Biochem Physiol 37:911–917. https://doi.org/10.1139/o59-099
Hoekman SK, Broch A, Robbins C, Ceniceros E, Natarajan M (2012) Review of biodiesel composition, properties, and specifications. Renew Sust Energ Rev 16:143–169. https://doi.org/10.1016/j.rser.2011.07.143
Van Wychen S, Ramirez K, Laurens LM (2013) Determination of total lipids as fatty acid methyl esters (FAME) by in situ transesterification. Technical Report NREL/TP-5100-60958. National Renewable Energy Laboratory, 15013 Denver West Parkway
Li J, Li C, Lan CQ, Liao D (2018) Effects of sodium bicarbonate on cell growth, lipid accumulation, and morphology of Chlorella vulgaris. Microb Cell Factories 17:1–10. https://doi.org/10.1186/s12934-018-0953-4
Sakarika M, Kornaros M (2017) Kinetics of growth and lipids accumulation in Chlorella vulgaris during batch heterotrophic cultivation: effect of different nutrient limitation strategies. Bioresour Technol 243:356–365. https://doi.org/10.1016/j.biortech.2017.06.110
Sharma J, Kumar SS, Bishnoi NR, Pugazhendhi A (2018) Enhancement of lipid production from algal biomass through various growth parameters. J Mol Liq 269:712–720. https://doi.org/10.1016/j.molliq.2018.08.103
Xia L, Yang H, He Q, Hu C (2015) Physiological responses of freshwater oleaginous microalgae Desmodesmus sp. NMX451 under nitrogen deficiency and alkaline pH-induced lipid accumulation. J Appl Phycol 27:649–659. https://doi.org/10.1007/s10811-014-0371-x
Tiwari ON, Bhunia B, Muthuraj M et al (2020) Optimization of process parameters on lipid biosynthesis for sustainable biodiesel production and evaluation of its fuel characteristics. Fuel 269:117471. https://doi.org/10.1016/j.fuel.2020.117471
Nordstrom DK, Ball JW, McCleskey BR (2005) Ground water to surface water: chemistry of thermal outflows in Yellowstone National Park. In: Inskeep W, McDermott TR (eds) Geothermal biology and geochemistry in Yellowstone National Park. Montana State University, Bozeman, pp 73–94
Bajwa K, Bishnoi NR, Kirrolia A, Gupta S, Tamil Selvan S (2019) Response surface methodology as a statistical tool for optimization of physio-biochemical cellular components of microalgae Chlorella pyrenoidosa for biodiesel production. Appl Water Sci 9. https://doi.org/10.1007/s13201-019-0969-x
Morschett H, Freier L, Rohde J, Wiechert W, von Lieres E, Oldiges M (2017) A framework for accelerated phototrophic bioprocess development: integration of parallelized microscale cultivation, laboratory automation and Kriging-assisted experimental design. Biotechnol Biofuels 10:1–13. https://doi.org/10.1186/s13068-017-0711-6
Srinivasan R, Mageswari A, Subramanian P, Suganthi C, Chaitanyakumar A, Aswini V, Gothandam KM (2018) Bicarbonate supplementation enhances growth and biochemical composition of Dunaliella salina V−101 by reducing oxidative stress induced during macronutrient deficit conditions. Sci Rep 8:1–14. https://doi.org/10.1038/s41598-018-25417-5
Van Mooy BAS, Rocap G, Fredricks HF et al (2006) Sulfolipids dramatically decrease phosphorus demand by picocyanobacteria in oligotrophic marine environments. Proc Natl Acad Sci U S A 103:8607–8612. https://doi.org/10.1073/pnas.0600540103
Knothe G (2009) Improving biodiesel fuel properties by modifying fatty ester composition. Energy Environ Sci 2:759–766. https://doi.org/10.1039/b903941d
Acknowledgements
The authors are thankful to the Head, Department of Botany and Microbiology, H.N.B. Garhwal University, for providing necessary facilities. PS thanks the University Grants Commission, New Delhi, for financial assistance in the form of a University Research Fellowship and CSIR-Indian Institute of Petroleum, Dehradun, for GC-MS analysis.
Funding
1. University Grants Commission, New Delhi, India
2. Department of Botany and Microbiology, Hemvati Nandan Bahuguna Garhwal University, Srinagar Garhwal, India
Author information
Authors and Affiliations
Contributions
PS planned and executed experiments, prepared a draft of the manuscript, and finalized the manuscript for submission. D. Kumar was involved in conceiving the idea and planning of the experiments. Being the supervisor of the first and corresponding author, he was responsible for the organization of lab and experimental facilities for the work contained in the paper.
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic Supplementary Material
Supplementary Fig. 1
Perturbation plots of factors influencing biomass productivity (a) and lipid content (b) of L. foveolorum HNBGU-001 [A: nitrate-N, B: phosphate-P, C: sulphate-S, D: carbonate-C, E: Ca2+, F: Fe3+, K: Dummy factor]. (PNG 344 kb).
Rights and permissions
About this article
Cite this article
Singh, P., Kumar, D. Biomass and Lipid Productivities of Cyanobacteria- Leptolyngbya foveolarum HNBGU001. Bioenerg. Res. 14, 278–291 (2021). https://doi.org/10.1007/s12155-020-10170-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12155-020-10170-3