Abstract
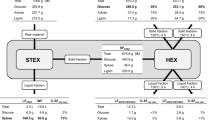
Ethanol organosolv (EO) fractionation is a promising technology for producing high-purity lignin from kenaf, a lignocellulosic biomass. However, EO fractionation could not be used for the hemicellulose fraction because of the removal of hemicellulose into black liquor, which is a major drawback. Dilute hydrochloric acid (DHA) fractionation prior to EO fractionation has been proposed as a strategy to overcome this problem. In this study, the hemicellulose fraction of kenaf was maximally fractioned using 0.5% (w/w) HCl at 145 °C for 60 min. Subsequently, the non-washed and dried fibers, the cellulose-lignin fraction, were also fractionated to extract lignin using 60% (v/v) ethanol at 180 °C for 60 min through an optimization process. In each fractionation process, hemicellulose and lignin were removed at 86.11 and 68.86 % from untreated kenaf, respectively. Thus, DHA and EO fractionations are effective procedures to separate the lignin (94.73% purity) and hemicellulosic hydrolysate (19.65 g/L sugar) fractions in addition to cellulose-rich solid (glucan content 80.05%).





Similar content being viewed by others
References
Yun G (2016) China’s response to climate change issues after Paris Climate Change Conference. Adv Clim Chang Res 7:235–240. https://doi.org/10.1016/j.accre.2016.10.001
Bhutto AW, Bazmi AA, Zahedi G (2011) Green energy: issues and challenges for Pakistan-biomass energy prospective. Renew Sust Energ Rev 15:3207–3219. https://doi.org/10.1016/j.rser.2011.04.015
Ching A, Webber III CL, Neill SW. (1993) The effect of location and cultivar on kenaf yield components. Ind Crop Prod 2:27–31. https://doi.org/10.1016/0926-6690(92)90018-Q
Francosi LE, Donovan TJ, Yield Mass EV (1992) Vegetative growth and fiber length of kenaf grown on saline soil. Agron J 88:592–528. https://doi.org/10.2134/agronj1992.00021962008400040010x
Saba N, Jawaid M, Hakeem KR, Paridah MT, Khalina A, Alothman OY (2015) Potential of bioenergy production from industrial kenaf (Hibiscus cannabinus L.) based on Malaysian perspective. Renew Sust Energ Rev 42:446–459. https://doi.org/10.1016/j.rser.2014.10.029
Lam TBT, Hori K, Iiyama K (2003) Structural characteristics of cell walls of kenaf (Hibiscus cannabinus L.) and fixation of carbon dioxide. J Wood Sci 49(3):255–261. https://doi.org/10.1007/s10086-002-0469-7
Bootsma JA (2016) Recovery of monosaccharides via catalytic hydrolysis. Iowa State University, Dissertation
Li P, Cai D, Zhang C, Li S, Qin P, Chen C, Wang Y, Wang Z (2016) Comparison of two-stage acid-alkali and alkali-acid pretreatments on enzymatic saccharification ability of the sweet sorghum fiber and their physicochemical characterizations. Bioresour Technol 221:636–644. https://doi.org/10.1016/j.biortech.2016.09.075
Liu Q, Li WZ, Ma QZ, An SX, Li MH, Jameel H, Chang HM (2016) Pretreatment of corn stover for sugar production using a two-stage dilute acid followed by wet-milling pretreatment process. Bioresour Technol 211:435–442. https://doi.org/10.1016/j.biortech.2016.03.131
Zu S, Li WZ, Zhang MJ, Wang Z, Jameel H, Chang HM (2014) Pretreatment of corn stover for sugar production using dilute hydrochloric acid followed by lime. Bioresour Technol 152:364–370. https://doi.org/10.1016/j.biortech.2013.11.034
Jang SK, Kim HY, Jeong HS, Kim JY, Yeo HM, Choi IG (2016) Effect of ethanol organosolv pretreatment factors on enzymatic digestibility and ethanol organosolv lignin structure from Liriodendron tulipifera in specific combined severity factors. Renew Energy 87:599–606. https://doi.org/10.1016/j.renene.2015.10.045
Zhao X, Cheng K, Liu D (2009) Organosolv pretreatment of lignocellulosic biomass for enzymatic hydrolysis. Appl Microbiol Biotechnol 82:815–827. https://doi.org/10.1007/s00253-009-1883-1
Amin H, Karimi K, Zilouei H (2014) Organosolv pretreatment of rice straw for efficient acetone, butanol, and ethanol production. Bioresour Technol 152:450–456. https://doi.org/10.1016/j.biortech.2013.11.038
Brodeur G, Telotte J, Stickel JJ, Ramakrishnan S (2016) Two-stage dilute-acid and organic-solvent lignocellulosic pretreatment for enhanced bioprocessing. Bioresour Technol 220:621–628. https://doi.org/10.1016/j.biortech.2016.08.089
Kim JW, Kim KS, Lee JS, Park SM, Cho HW, Park JC, Kim JS (2011) Two-stage pretreatment of rice straw using aqueous ammonia and dilute acid. Bioresour Technol 102:8992–8999. https://doi.org/10.1016/j.biortech.2011.06.068
Kärcher MA, Iqbal Y, Lewandowski I, Senn T (2016) Efficiency of single stage- and two stage pretreatment in biomass with different lignin content. Bioresour Technol 211:787–791. https://doi.org/10.1016/j.biortech.2016.04.017
Li W, Liu Q, Ma Q, Zhang T, Ma L, Jameel H, Chang HM (2016) A two-stage pretreatment process using dilute hydrochloric acid followed by Fenton oxidation to improve sugar recovery from corn stover. Bioresour Technol 219:753–756. https://doi.org/10.1016/j.biortech.2016.08.025
Moodley P, Gueguim Kana EB (2017) Comparison of a two-stage and a combined single stage salt-acid based lignocellulosic pretreatment for enhancing enzymatic saccharification. Ind Crop Prod 108:219–224. https://doi.org/10.1016/j.indcrop.2017.06.048
Ravindran R, Jaiswal S, Abu-Ghannam N, Jaiswal AK (2017) Two-step sequential pretreatment for the enhanced enzymatic hydrolysis of coffee spent waste. Bioresour Technol 239:276–284. https://doi.org/10.1016/j.biortech.2017.05.049
Kim DY, Kim YS, Kim TH, Oh KK (2016) Two-stage, acetic acid-aqueous ammonia, fractionation of empty fruit bunches for increased lignocellulosic biomass utilization. Bioresour Technol 199:121–127. https://doi.org/10.1016/j.biortech.2015.09.049
Kim SJ, Um BH (2015) Comparison of hemicellulose extracts from two pulping woodchips with green liquor followed by scale-up pre-hemicellulose extraction. Appl Biochem Biotechnol 175(5):2501–2515. https://doi.org/10.1007/s12010-014-1408-y
Ko JK, Bak JS, Jung MW, Lee HJ, Choi IG, Kim TH, Kim KH (2009) Ethanol production from rice straw using optimized aqueous-ammonia soaking pretreatment and simultaneous saccharification and fermentation processes. Bioresour Technol 100:4374–4380. https://doi.org/10.1016/j.biortech.2009.04.026
Kim SJ, Kim TH, Oh KK (2018) Deacetylation followed by fractionation of yellow poplar sawdust for the production of toxicity-reduced hemicellulosic sugar for ethanol fermentation. Energies. 11(2):404. https://doi.org/10.3390/en11020404
Kim SJ, Um BH, Im DJ, Lee JH, Oh KK (2018) Combined ball milling and ethanol organosolv pretreatment to improve the enzymatic digestibility of three types of herbaceous biomass. Energies. 11(9):2457. https://doi.org/10.3390/en11092457
Vroom KE (1957) The H factor: a means of expressing cooking times and temperatures as a single variable, Pulp and Paper Mag. Can. 58(3):228–231
Kim YS, Jang JY, Park SJ, Um BH (2018) Dilute sulfuric acid fractionation of Korean food waste for ethanol and lactic acid production by yeast. Waste Manag 74:231–240. https://doi.org/10.1016/j.wasman.2018.01.012
Sluiter A, Hames B, Ruiz R, Scarlata C, Sluiter J, Tmpleton D, (2008) Determination of ash in biomass. National Renewable Energy Laboratory, CO, Golden, NREL/TP-510–42622.
Sluiter A, Hames B, Ruiz R, Scarlata C, Sluiter J, Tmpleton D, (2008) Determination of sugars, byproducts, and degradation products in liquid fraction process samples. National Renewable Energy Laboratory, CO, Golden, NREL/TP-510–42623.
Sluiter A, Ruiz R, Scarlata C, Sluiter J, Tmpleton D, (2008) Determination of extractives in biomass. National Renewable Energy Laboratory, CO, Golden, NREL/TP-510–42619.
Sluiter A, Hames B, Ruiz R, Scarlata C, Sluiter J, Tmpleton D, Crocker D, (2012) Determination of structural carbohydrates and lignin in biomass. National Renewable Energy Laboratory, CO, Golden, NREL/TP-510–42618.
Kumar P, Barrett DM, Delwiche MJ, Stroeve P (2009) Methods for pretreatment of lignocellulosic biomass for efficient hydrolysis and biofuel production. Ind Eng Chem Res 48:3713–3729. https://doi.org/10.1021/ie801542g
Um BH, van Walsum GP (2012) Effect of pretreatment severity on accumulation of major degradation products from dilute acid pretreated corn stover and subsequent inhibition of enzymatic hydrolysis of cellulose. Appl Biochem Biotechnol 168(2):406–420. https://doi.org/10.1007/s12010-012-9784-7
Pandey A, Negi S, Binod P, Larroche C, (2014) Pretreatment of biomass: process and technologies. ELSEVIER, 1st Edition, Chapter 5, pp 61
Choi WI, Ryu HJ, Kim SJ, Oh KK (2017) Thermo-mechanical fractionation of yellow poplar sawdust with a low reaction severity using continuous twin screw-driven reactor for high hemicellulosic sugar recovery. Bioresour Technol 241:63–69. https://doi.org/10.1016/j.biortech.2017.05.089
Gandla ML, Martin C, Jonsson LJ (2018) Analytical enzymatic saccharification of lignocellulosic biomass for conversion to biofuels and bio-based chemicals. Energies. 11:2936. https://doi.org/10.3390/en11112936
Hua DR, Wu YL, Liu YF, Chen Y, Yang MD, Lu XN, Li J (2016) Preparation of furfural and reaction kinetics of xylose dehydration to furfural in high-temperature water. PETROL SCI 13(1):167–172. https://doi.org/10.1007/s12182-015-0069-y
Yoo CG, Lee CW, Kim TH (2011) Optimization of two stage fractionation process for lignocellulosic biomass using response surface methodology (RSM). Biomass Bioenergy 35:4901–4909. https://doi.org/10.1016/j.biombioe.2011.10.015
Li T, Remon J, Jiang Z, Budarin VL, Clark JH (2018) Towards the development of a novel “bamboo-refinery” concept: selective bamboo fractionation by means of a microwave-assisted, acid-catalysed, organosolv process. Energy Convers Manage 155:147–160. https://doi.org/10.1016/j.enconman.2017.10.077
Shui T, Feng S, Yuan Z, Kuboki T, Xu CC (2016) Highly efficient organosolv fractionation of cornstalk into cellulose and lignin in organic acids. Bioresour Technol 218:953–961. https://doi.org/10.1016/j.biortech.2016.07.054
Panagiotopoulos IA, Chandra RP, Saddler JN (2013) A two-stage pretreatment approach to maximize sugar yield and enhance reactive lignin recovery from poplar wood chips. Bioresour Technol 130:570–577. https://doi.org/10.1016/j.biortech.2012.12.093
Shen D, Xiao R, Gu S, Zhang H, (2013) An overview of thermal decomposition of cellulose in lignocellulosic biomass. Chapter 9, in: T. van der Ven, J. Kadla, Intech (Eds.), cellulose-biomass conversion, agricultural biological sciences, Intech – open sciences.
Kim SJ, Um BH (2018) Delignification from Geodae-Ukase1 using soda-pulping followed by evaluation on recycling of liquid-liquid extraction solvent. BIOMASS BIOENERG 109:23–30. https://doi.org/10.1016/j.biombioe.2017.12.003
Hashmi SF, Merio-Talvio H, Hakonen KJ, Ruuttunen K, Sixta H (2017) Hydrothermolysis of organosolv lignin for the production of bio-oil rich in monoaromatic phenolic compounds. Fuel Process Technol 168:74–83. https://doi.org/10.1016/j.fuproc.2017.09.005
Hu Y, Wang S, Wang Q, He Z, Lin X, Xu S, Ji H, Li Y (2017) Effect of different pretreatments on the thermal degradation of seaweed biomass. P COMBUST INST 36:2271–2281. https://doi.org/10.1016/j.proci.2016.08.086
Martinez-Patino JC, Lu-Chai TA, Gullon B, Ruiz E, Romero I, Castro E, Lema JM (2018) Application of a combined fungal and diluted acid pretreatment on olive tree biomass. Ind Crop Prod 121:10–17. https://doi.org/10.1016/j.indcrop.2018.04.078
Funding
This work was financially supported by the Korea Institute of Energy Technology Evaluation and Planning (KETEP) and the Ministry of Trade, Industry and Energy (MOTIE) of the Republic of Korea (No. 20173030091900).
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Kim, S.J., Um, B.H. Process Variable Optimization of Kenaf Two-Stage Fractionation Based on Dilute Hydrochloric Acid Followed by Ethanol Organosolv for Component Separation. Bioenerg. Res. 13, 249–259 (2020). https://doi.org/10.1007/s12155-019-10057-y
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12155-019-10057-y