Abstract
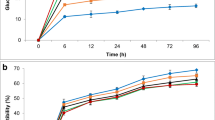
This study reports on the evaluation of a two-stage pretreatment process for preparing cotton gin trash (CGT) for conversation to ethanol. During the first stage, CGT was mixed with acid at 12% H2SO4 on solids and heated to 180 °C for 15 min in a pressurised stirred reactor. Pressed first-stage pretreated fibres were heated to 200 °C for 5 min during the second stage. The two-stage process facilitated excellent sugar recovery from both cellulose (84%) and hemicellulose (78%) fractions of CGT. Recombinant yeast GSF335 propagated on first-stage liquors yielded 41.8 g kg−1 of dry CGT and was compared with the commercial yeast during separate hydrolysis and fermentation (SHF) and simultaneous saccharification and fermentations (SSF). SHF of pretreated CGT fibre yielded the equivalent of 66.2 and 64.0 kg ethanol per tonne of unprocessed CGT with GSF335 and the commercial yeast, respectively. SSF ethanol yields for the commercial yeast were significantly lower (50.3 kg) while GSF335 correspondingly produced 63.8 kg ethanol per tonne of raw CGT.




Similar content being viewed by others
References
Hassall and Associates (2005) Value of research investment relating to the waste classification of cotton gin trash. Cotton Research and Development Corporation
Anon (2018) 2018 Cotton Annual - Statics booklet. Avaiable from http://cottonaustralia.com.au/cotton-library/publications/brochures. http://cottonaustralia.com.au/cotton-library/statistics. Accessed 12 Jun 2018
Hamawand I, Sandell G, Pittaway P, Chakrabarty S, Yusaf T, Chen GN, Seneweera S, Al-Lwayzy S, Bennett J, Hopf J (2016) Bioenergy from cotton industry wastes: a review and potential. Renew Sust Energ Rev 66:435–448
Egbuta MA, McIntosh S, Waters DLE, Vancov T, Liu L (2017) Biological importance of cotton by-products relative to chemical constituents of the cotton plant. Molecules 22(1):25
McIntosh S, Vancov T, Palmer J, Morris S (2014) Ethanol production from cotton gin trash using optimised dilute acid pretreatment and whole slurry fermentation processes. Bioresour Technol 173:42–51
Agblevor FA, Batz S, Trumbo J (2003) Composition and ethanol production potential of cotton gin residues. Appl Biochem Biotechnol 105(1–3):219–230
Jeoh T, Agblevor FA (2001) Characterization and fermentation of steam exploded cotton gin waste. Biomass Bioenergy 21(2):109–120
Sahu S, Pramanik K (2018) Evaluation and optimization of organic acid pretreatment of cotton gin waste for enzymatic hydrolysis and bioethanol production. Appl Biochem Biotechnol 186:1047–1060. https://doi.org/10.1007/s12010-018-2790-7
Shen J, Agblevor FA (2008) Kinetics of enzymatic hydrolysis of steam-exploded cotton gin waste. Chem Eng Commun 195(9):1107–1121
Silverstein RA, Chen Y, Sharma-Shivappa RR, Boyette MD, Osborne J (2007) A comparison of chemical pretreatment methods for improving saccharification of cotton stalks. Bioresour Technol 98(16):3000–3011
Inoue H, Fujimoto S, Sakaki T (2016) Two-step hot-compressed water treatment of Douglas fir for efficient total sugar recovery by enzymatic hydrolysis. BioResources 11(2):5124–5137
Vancov T, Palmer J, Keen B (2018) A two stage pretreatment process to maximise recovery of sugars from cotton gin trash. Bioresour Technol Rep 4:114–122
Kwak S, Jin YS (2017) Production of fuels and chemicals from xylose by engineered Saccharomyces cerevisiae: a review and perspective. Microb Cell Factories 16:15
Jansen MLA, Bracher JM, Papapetridis I, Verhoeven MD, de Bruijn H, de Waal PP, van Maris AJA, Klaassen P, Pronk JT (2017) Saccharomyces cerevisiae strains for second-generation ethanol production: from academic exploration to industrial implementation. FEMS Yeast Res 17(5):20
Hou J, Qiu CX, Shen Y, Li HX, Bao XM (2017) Engineering of Saccharomyces cerevisiae for the efficient co-utilization of glucose and xylose. FEMS Yeast Res 17(4):11
Demeke MM, Dumortier F, Li Y, Broeckx T, Foulquie-Moreno MR, Thevelein JM (2013) Combining inhibitor tolerance and D-xylose fermentation in industrial Saccharomyces cerevisiae for efficient lignocellulose-based bioethanol production. Biotechnol Biofuels 6(1):120
Sluiter A, Hames B, Ruiz R, Scarlata C, Sluiter J, Templeton D, Crocker D (2008) Determination of structural carbohydrates and lignin in biomass. National Renewable Energy Laboratory
Adney B, Baker J (2008) Measurement of cellulase activity: NREL technical report NREL/TP-510-42628
Jeon Y, Xun Z, Rogers P (2011) Comparative evaluations of cellulosic raw materials for second generation bioethanol production. Lett Appl Microbiol 51:518–524
Gupta A, Verma JP (2015) Sustainable bio-ethanol production from agro-residues: a review. Renew Sust Energ Rev 41:550–567
Vancov T, McIntosh S (2011) Alkali pretreatment of cereal crop residues for second-generation biofuels. Energy Fuel 25(7):2754–2763
McIntosh S, Vancov T, Palmer J, Spain M (2012) Ethanol production from eucalyptus plantation thinnings. Bioresour Technol 110:264–272
Fockink DH, Maceno MAC, Ramos LP (2015) Production of cellulosic ethanol from cotton processing residues after pretreatment with dilute sodium hydroxide and enzymatic hydrolysis. Bioresour Technol 187:91–96
Vancov T, McIntosh S (2011) Effects of dilute acid pretreatment on enzyme saccharification of wheat stubble. J Chem Technol Biotechnol 86(6):818–825
Shi J, Chinn MS, Sharma-Shivappa RR (2008) Microbial pretreatment of cotton stalks by solid state cultivation of Phanerochaete chrysosporium. Bioresour Technol 99(14):6556–6564
Plácido J, Imam T, Capareda S (2013) Evaluation of ligninolytic enzymes, ultrasonication and liquid hot water as pretreatments for bioethanol production from cotton gin trash. Bioresour Technol 139:203–208
Ko JK, Um Y, Park YC, Seo JH, Kim KH (2015) Compounds inhibiting the bioconversion of hydrothermally pretreated lignocellulose. Appl Microbiol Biotechnol 99(10):4201–4212
Duarte G, Moreira L, Jaramillo P, Filho E (2012) Biomass-derived inhibitors of holocellulases. BioEnergy Res 5(3):768–777
Chang VS, Holtzapple MT (2000) Fundamental factors affecting biomass enzymatic reactivity. Appl Biochem Biotechnol 84-86:5–37
Jian S, Dong W, Libing Z, SB A, Seema S, Bin Y, WC E (2017) Dynamic changes of substrate reactivity and enzyme adsorption on partially hydrolyzed cellulose. Biotechnol Bioeng 114(3):503–515
Bakker BM, Overkamp KM, van Maris AJA, Kötter P, Luttik MAH, van Dijken JP, Pronk JT (2001) Stoichiometry and compartmentation of NADH metabolism in Saccharomyces cerevisiae. FEMS Microbiol Rev 25(1):15–37
D'Amore T, Panchal CJ, Russell I, Stewart GG (1990) A study of ethanol tolerance in yeast. Crit Rev Biotechnol 9(4):287–304
Casey GP, Ingledew WM (1986) Ethanol tolerance in yeasts. Crit Rev Microbiol 13(3):219–280
Ingledew WM (2009) Yeasts: physiology, nutrition and ethanol production. In: Ingledew WM, Kelsall DR, Austin GD, Kluhspies C (eds) The alcohol textbook : a reference for the beverage, fuel and industrial alcohol industries, 5th edn. Nottingham University Press, Nottingham, pp 101–113
Bezerra RM, Dias AA (2005) Enzymatic kinetic of cellulose hydrolysis: inhibition by ethanol and cellobiose. Appl Biochem Biotechnol 126(1):49–59
Wu Z, Lee YY (1997) Inhibition of the enzymatic hydrolysis of cellulose by ethanol. Biotechnol Lett 19(10):977–979
Manfredi AP, Ballesteros I, Sáez F, Perotti NI, Martínez MA, Negro MJ (2018) Integral process assessment of sugarcane agricultural crop residues conversion to ethanol. Bioresour Technol 260:241–247
Cuevas M, Sánchez S, García JF, Baeza J, Parra C, Freer J (2015) Enhanced ethanol production by simultaneous saccharification and fermentation of pretreated olive stones. Renew Energy 74:839–847
Acknowledgements
The work was undertaken as part of the Biorefineries for Profit project which was funded by the Sugar Research Australia (SRA) and the Australian Government Department of Agriculture and Water Resources through the Rural R&D for Profit Program and Queensland Government Department of Agriculture and Fisheries, Cotton Research and Development Corporation, Forest and Wood Products Australia, Australian Pork Limited, Southern Oil Refining, Queensland University of Technology and NSW Department of Primary Industries, Australia. We express our gratitude to Yarraman Gin (Namoi Cotton Co-operative Ltd., NSW Australia) for supplying cotton-ginning residues.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Vancov, T., Palmer, J. & Keen, B. Two-Stage Pretreatment Process Validation for Production of Ethanol from Cotton Gin Trash. Bioenerg. Res. 12, 593–604 (2019). https://doi.org/10.1007/s12155-019-09989-2
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12155-019-09989-2