Abstract
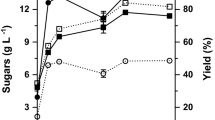
Orange peel waste was converted into ethanol by consecutive acid-catalyzed steam-explosion (ACSE), enzymatic saccharification, and fermentation with Saccharomyces cerevisiae F15. With the aim of increasing the concentration of fermentable sugars in the hydrolysate and to fully recycle the spent acid liquor as the suspending medium for saccharification, the technical feasibility of increasing the solid loading in the ACSE step from 160 to 480 g L−1 was assessed. At high solid loading in the ACSE pretreatment (HSLAP), the solubilization degrees of polysaccharides were lower while those of potential inhibitors (e.g., acetic and formic acids and phenols) were generally higher than those found at low solid loading (LSLAP). However, residual solids from both solid loadings showed similar susceptibility to enzymatic saccharification (ES) in a 7-L stirred-tank reactor (STR) (cellulase, 12 FPU g−1 cellulose; pectinase, 25 IU g−1 dry matter; 72 h incubation at 50 °C). Fermentation was performed in a 1-L STR along five repeated batches with the first one being used to enable yeast proliferation. By using the hydrolysates arising from the HSLAP-ES combination, it was possible to rely on a fermentation medium with a 2.5-fold higher concentration of simple sugars and to double the ethanol concentration in the final beer to be distilled of. However, the higher content of inhibitory compounds in hydrolysates from HSLAP-ES than in the LSLAP-ES ones led to a reduction in the ethanol yield per unit substrate consumed (0.49 vs. 0.41 g g−1, respectively) and overall productivity (3.4 vs. 2.7 g h−1, respectively).



Similar content being viewed by others
References
Marin FR, Soler-Rivas C, Benavente-Garcia O, Castillo J, Perez-Alvarez JA (2007) By-products from different citrus processes as a source of customized functional fibres. Food Chem 100(2):736–741. doi:10.1016/j.foodchem.2005.04.040
Crawshaw R (2004) Co-product feeds: animal feeds from the food and drinks industries. Nottingham University Press, Nottingham
Grohmann K, Baldwin EA (1992) Hydrolysis of orange peel with pectinase and cellulase enzymes. Biotechnol Lett 14(12):1169–1174. doi:10.1007/BF01027023
Grohmann K, Baldwin EA, Buslig BA (1994) Production of ethanol from enzymatically hydrolyzed orange peel by the yeast Saccharomyces cerevisiae. Appl Biochem Biotechnol 45–46(2):315–327. doi:10.1007/BF02941808
Grohmann K, Cameron RG, Buslig BS (1995) Fractionation and pretreatment of orange peel by dilute acid hydrolysis. Bioresour Technol 54(2):129–141. doi:10.1016/0960-8524(95)00121-2
Pourbafrani M, Forgács G, Horváth IS, Niklasson C, Taherzadeh MJ (2010) Production of biofuels, limonene and pectin from citrus wastes. Bioresour Technol 101(11):4246–4250. doi:10.1016/j.biortech.2010.01.077
Widmer W, Zhou W, Grohmann K (2010) Pretreatment effects on orange processing waste for making ethanol by simultaneous saccharification and fermentation. Bioresour Technol 101(14):5242–5249. doi:10.1016/j.biortech.2009.12.038
Wilkins MR, Widmer WW, Grohmann K (2007) Simultaneous saccharification and fermentation of citrus peel waste by Saccharomyces cerevisiae to produce ethanol. Process Biochem 42(2):1614–1619. doi:10.1016/j.procbio.2007.09.006
Zhou W, Widmer W, Grohmann K (2008) Developments in ethanol production from citrus peel waste. Proc Fla State Hortic Soc 121:307–310
Wilkins MR, Suryawati L, Maness NO, Chrz D (2007) Ethanol production by Saccharomyces cerevisiae and Kluyveromyces marxianus in the presence of orange-peel oil. World J Microbiol Biotechnol 23:1161–1168
Sandhu KS, Minhas K (2006) Oranges and citrus juices. In: Hui YH (ed) Handbook of fruits and fruit processing. Blackwell, Oxford, pp 309–358
Santi G, Crognale S, D’Annibale A, Petruccioli M, Ruzzi M, Valentini R, Moresi M (2014) Orange peel pretreatment in a novel lab-scale direct steam-injection apparatus for ethanol production. Biomass Bioenergy 61:146–156. doi:10.1016/j.biombioe.2013.12.007
Boluda-Aguilar M, García-Vidal L, del Pilar G-CF, López-Gómez A (2010) Mandarin peel wastes pretreatment with steam explosion for bioethanol production. Bioresour Technol 101(10):3506–3513. doi:10.1016/j.biortech.2009.12.063
Ghose TK (1987) Measurement of cellulase activities. Pure Appl Chem 59(2):257–268. doi:10.1351/pac198759020257
Collmer A, Ried JL, Mount MS (1988) Assay methods for pectic enzymes. In: Colowick SP, Kaplan N (eds) Methods in enzymology: biomass. Part B, vol. 161. Academic, San Diego, pp 329–335
Van Soest PJ (1963) Use of detergents in the analysis of fibrous feeds. II. A rapid method for the determination of fiber and lignin. J Assoc Off Anal Chem 46:829–835
Larsson S, Reimann A, Nilvebrant N, Jönsson LJ (1999) Comparison of different methods for the detoxification of lignocellulose hydrolyzates of spruce. Appl Biochem Biotechnol 77–79(1–3):91–103. doi:10.1385/ABAB:77:1-3:91
Swain T, Hillis WE (1959) The phenolic constituents of Prunus domestica. J Sci Food Agric 10(1):63–68. doi:10.1002/jsfa.2740100110
Taylor KA, Buchanan-Smith JG (1992) A colorimetric method for the quantitation of uronic acids and a specific assay for galacturonic acid. Anal Biochem 201(1):190–196. doi:10.1016/0003-2697(92)90194-C
Talebnia F, Pourbafrani M, Lundin M, Taherzadeh MJ (2008) Optimization study of citrus wastes saccharification by dilute-acid hydrolysis. Bioresources 3(1):108–122
Ximenes E, Kim Y, Mosier N, Dien B, Ladisch M (2010) Inhibition of cellulases by phenols. Enzym Microb Technol 46:170–176. doi:10.1016/j.enzmictec.2009.11.001
Palmqvist E, Hahn-Hägerdal B (2000) Fermentation of lignocellulosic hydrolysates. II: inhibitors and mechanisms of inhibition. Bioresour Technol 74(1):25–33. doi:10.1016/S0960-8524(99)00161-3
Oberoi HS, Vadlani PV, Madl RL, Saida L, Abeykoon JP (2010) Ethanol production from orange peels: two-stage hydrolysis and fermentation studies using optimized parameters through experimental design. J Agric Food Chem 58(6):3422–3429. doi:10.1021/jf903163t
Asghari FS, Yoshida H (2007) Kinetics of the decomposition of fructose catalyzed by hydrochloric acid in subcritical water: formation of 5-hydroxymethylfurfural, levulinic, and formic acids. Ind Eng Chem Res 46(23):7703–7710. doi:10.1021/ie061673e
Tsukamoto J, Durán N, Tasic L (2013) Nanocellulose and bioethanol production from orange waste using isolated microorganisms. J Braz Chem Soc 24(9):1537–1543. doi:10.5935/0103-5053.20130195
Hodge DB, Karim MN, Schell DJ, McMillan JD (2008) Soluble and insoluble solids contributions to high-solids enzymatic hydrolysis of lignocellulose. Bioresour Technol 99:8940–8948. doi:10.1016/j.biortech.2008.05.015
Kristensen JB, Felby C, Jørgensen H (2009) Yield-determining factors in high-solids enzymatic hydrolysis of lignocellulose. Biotechnol Biofuels 2:11. doi:10.1186/1754-6834-2-11
Timson DJ (2007) Galactose metabolism in Saccharomyces cerevisiae. Dyn Biochem Process Biotechnol Mol Biol 1(1):63–73
Leeper SA, Tsao GT (1987) Membrane separations in ethanol recovery: an analysis of two applications of hyperfiltration. J Membr Sci 30(3):289–312. doi:10.1016/S0376-7388(00)80124-
Acknowledgments
This research work was supported by a grant from the Italian Ministry of the Environment and Territories within the Italy-Israel Partnership in the Environmental Research and Development sector.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Santi, G., Jasiulewicz, J., Crognale, S. et al. High Solid Loading in Dilute Acid Hydrolysis of Orange Peel Waste Improves Ethanol Production. Bioenerg. Res. 8, 1292–1302 (2015). https://doi.org/10.1007/s12155-015-9591-4
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12155-015-9591-4