Abstract
Objective
We examined the diagnostic value of brain perfusion single-photon emission computed tomography (SPECT) using voxel-based statistical analysis with CT-based attenuation correction (CT-AC) by comparing it to that with Chang’s AC in mild cognitive impairment (MCI) patients and attempted to locate brain areas that are good indicators predicting the progression of MCI.
Methods
Twenty-six individuals matched for age, educational background and initial Mini-Mental State Examination (MMSE) score of more than 24 underwent SPECT with N-isopropyl-4-[123I]iodoamphetamine and were assigned to 2 groups: the stable MCI (S-MCI) group comprising 11 subjects who maintained their MMSE score (mean 27.0) during at least a 1-year follow-up period (mean 37.2 months) and the progressive MCI (P-MCI) group comprising 15 subjects whose MMSE scores decreased by 3 or more points (from 26.4 to 21.4, mean). The diagnostic values of the two AC methods for discriminating P-MCI from S-MCI were compared using voxel-based statistical analysis in the lobe (Level 2) and lobule/gyrus levels (Level 3).
Results
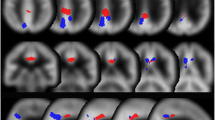
Receiver operating characteristic analysis revealed that the area under the curve (AUC) was higher with CT-AC than with Chang’s AC in the left temporal and limbic lobes in Level 2. In Level 3, the AUC in the left middle temporal gyrus was higher with CT-AC (0.852) than with Chang’s AC (0.827). There were differences between the gyri/lobules that showed higher AUCs with CT-AC and those that showed higher AUCs with Chang’s AC. When the gyri with the 4 highest AUCs were combined, AUC (0.897) and accuracy (84.6%) were better with CT-AC than with Chang’s AC (0.806 and 80.8%). Surprisingly, the AUCs in the posterior cingulate gyrus and precuneus, excluding the AUC in the right precuneus with Chang’s AC (0.715), were no more than 0.70 and less useful.
Conclusions
CT-AC may allow brain perfusion SPECT to reflect more exact neuropathic changes in MCI that would cause progression of early AD. CT-AC in conjunction with voxel-based statistical analysis could possess higher diagnostic accuracy for exacerbation of disease implying early Alzheimer changes in MCI patients, with decreases in cerebral perfusion in the left temporal and limbic lobes representing good indicators.




Similar content being viewed by others
Change history
26 March 2023
A Correction to this paper has been published: https://doi.org/10.1007/s12149-023-01831-2
References
Matsuda H. Role of neuroimaging in Alzheimer's disease, with emphasis on brain perfusion SPECT. J Nucl Med. 2007;48(8):1289–300. https://doi.org/10.2967/jnumed.106.037218.
Kaneta T, Nakatsuka M, Nakamura K, Seki T, Yamaguchi S, Tsuboi M, et al. Improved diagnostic accuracy of SPECT through statistical analysis and the detection of hot spots at the primary sensorimotor area for the diagnosis of Alzheimer disease in a community-based study: "The Osaki-Tajiri Project". Clin Nucl Med. 2016;41(1):e1–e6. https://doi.org/10.1097/rlu.0000000000000976.
Mizumura S, Kumita S, Cho K, Ishihara M, Nakajo H, Toba M, et al. Development of quantitative analysis method for stereotactic brain image: assessment of reduced accumulation in extent and severity using anatomical segmentation. Ann Nucl Med. 2003;17(4):289–95.
Mizumura S, Kumita S. Stereotactic statistical imaging analysis of the brain using the easy Z-score imaging system for sharing a normal database. Radiat Med. 2006;24(7):545–52. https://doi.org/10.1007/s11604-006-0056-8.
Matsuda H. The role of neuroimaging in mild cognitive impairment. Neuropathology. 2007;27(6):570–7. https://doi.org/10.1111/j.1440-1789.2007.00794.x.
Matsuda H, Mizumura S, Nagao T, Ota T, Iizuka T, Nemoto K, et al. Automated discrimination between very early Alzheimer disease and controls using an easy Z-score imaging system for multicenter brain perfusion single-photon emission tomography. AJNR Am J Neuroradiol. 2007;28(4):731–6.
Ishiwata A, Sakayori O, Minoshima S, Mizumura S, Kitamura S, Katayama Y. Preclinical evidence of Alzheimer changes in progressive mild cognitive impairment: a qualitative and quantitative SPECT study. Acta Neurol Scand. 2006;114(2):91–6. https://doi.org/10.1111/j.1600-0404.2006.00661.x.
Stodilka RZ, Kemp BJ, Prato FS, Nicholson RL. Importance of bone attenuation in brain SPECT quantification. J Nucl Med. 1998;39(1):190–7.
Van Laere K, Koole M, Versijpt J, Dierckx R. Non-uniform versus uniform attenuation correction in brain perfusion SPET of healthy volunteers. Eur J Nucl Med. 2001;28(1):90–8.
Petersen RC. Mild cognitive impairment as a diagnostic entity. J Intern Med. 2004;256(3):183–94. https://doi.org/10.1111/j.1365-2796.2004.01388.x.
McKhann GM, Knopman DS, Chertkow H, Hyman BT, Jack CR Jr, Kawas CH, et al. The diagnosis of dementia due to Alzheimer's disease: recommendations from the National Institute on Aging-Alzheimer's Association workgroups on diagnostic guidelines for Alzheimer's disease. Alzheimers Dement. 2011;7(3):263–9. https://doi.org/10.1016/j.jalz.2011.03.005.
Minoshima S, Foster NL, Kuhl DE. Posterior cingulate cortex in Alzheimer's disease. Lancet. 1994;8926:895.
Braak H, Alafuzoff I, Arzberger T, Kretzschmar H, Del Tredici K. Staging of Alzheimer disease-associated neurofibrillary pathology using paraffin sections and immunocytochemistry. Acta Neuropathol. 2006;112(4):389–404. https://doi.org/10.1007/s00401-006-0127-z.
Farid K, Petras S, Poullias X, Caillat-Vigneron N. Clinical impact of nonuniform CT-based attenuation correction in brain perfusion SPECT/CT using (99m)Tc-ECD. Clin Nucl Med. 2014;39(6):e343–e345345. https://doi.org/10.1097/rlu.0000000000000320.
Braak H, Braak E. Diagnostic criteria for neuropathologic assessment of Alzheimer's disease. Neurobiol Aging. 1997;18(4 Suppl):S85–S8888.
Jack CR Jr, Knopman DS, Jagust WJ, Petersen RC, Weiner MW, Aisen PS, et al. Tracking pathophysiological processes in Alzheimer's disease: an updated hypothetical model of dynamic biomarkers. Lancet Neurol. 2013;12(2):207–16. https://doi.org/10.1016/s1474-4422(12)70291-0.
Maruyama M, Shimada H, Suhara T, Shinotoh H, Ji B, Maeda J, et al. Imaging of tau pathology in a tauopathy mouse model and in Alzheimer patients compared to normal controls. Neuron. 2013. https://doi.org/10.1016/j.neuron.2013.07.037.
Mosconi L, Tsui WH, Herholz K, Pupi A, Drzezga A, Lucignani G, et al. Multicenter standardized 18F-FDG PET diagnosis of mild cognitive impairment, Alzheimer's disease, and other dementias. J Nucl Med. 2008;49(3):390–8. https://doi.org/10.2967/jnumed.107.045385.
Mosconi L, Mistur R, Switalski R, Tsui WH, Glodzik L, Li Y, et al. FDG-PET changes in brain glucose metabolism from normal cognition to pathologically verified Alzheimer's disease. Eur J Nucl Med Mol Imaging. 2009;36(5):811–22. https://doi.org/10.1007/s00259-008-1039-z.
de Leon MJ, Convit A, Wolf OT, Tarshish CY, DeSanti S, Rusinek H, et al. Prediction of cognitive decline in normal elderly subjects with 2-[18F]fluoro-2-deoxy-d-glucose/positron-emission tomography (FDG/PET). Proc Natl Acad Sci USA. 2001;98(19):10966–71. https://doi.org/10.1073/pnas.191044198.
Foster NL, Wang AY, Tasdizen T, Fletcher PT, Hoffman JM, Koeppe RA. Realizing the potential of positron emission tomography with 18F-fluorodeoxyglucose to improve the treatment of Alzheimer's disease. Alzheimers Dement. 2008;4(Suppl 1):S29–S36. https://doi.org/10.1016/j.jalz.2007.10.004.
Alafuzoff I, Arzberger T, Al-Sarraj S, Bodi I, Bogdanovic N, Braak H, et al. Staging of neurofibrillary pathology in Alzheimer's disease: a study of the BrainNet Europe Consortium. Brain Pathol. 2008;18(4):484–96.
Inui Y, Ichihara T, Uno M, Ishiguro M, Ito K, Kato K, et al. CT-based attenuation correction and resolution compensation for I-123 IMP brain SPECT normal database: a multicenter phantom study. Ann Nucl Med. 2018;32(5):311–8. https://doi.org/10.1007/s12149-018-1248-x.
Yamazaki T, Inui Y, Ichihara T, Uno M, Ota S, Toyoda A, et al. Clinical utility of the normal database of (123)I-iodoamphetamine brain perfusion single photon emission computed tomography for statistical analysis using computed tomography-based attenuation correction: a multicenter study. Ann Nucl Med. 2019;33(11):835–41. https://doi.org/10.1007/s12149-019-01395-0.
Acknowlegements
We would like to thank the radiology technicians in the Division of Nuclear Medicine at the Department of Radiology for their valuable support: Kyoji Asano, Masaya Suda, Shinjiro Yoshida, Junya Tashiro, Satoshi Harashina and Toshio Maki.
Funding
This research did not receive any specific grant from funding agencies.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors have no competing interests to declare.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. This study was approved by the Nippon Medical School Ethics Committee.
Informed consent
Informed consent was obtained in the form of opt-out on the web-site. Those who rejected were excluded.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Sohara, K., Kiriyama, T., Mizumura, S. et al. Diagnostic utility and characteristics of CT-based attenuation correction in brain perfusion SPECT/CT in predicting the exacerbation of Alzheimer changes from mild cognitive impairment utilizing voxel-based statistical analysis in comparison with Chang’s method. Ann Nucl Med 34, 502–511 (2020). https://doi.org/10.1007/s12149-020-01477-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12149-020-01477-4