Abstract
Objectives
To compare the ablation results, therapeutic responses and adverse reactions between a low dose (1.1 GBq) or high dose (3.7 GBq) of 131I in low-/intermediate-risk differentiated thyroid cancer (DTC) patients. The factors influencing the ablation result and therapeutic response were also analyzed.
Methods
The researchers used a random number table to randomly assign the enrolled patients to the low-dose group or high-dose group at a 1:1 ratio, and assessment of ablation result, therapeutic response, and adverse reactions evaluated 6 ± 3 months after therapy.
Results
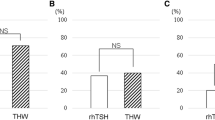
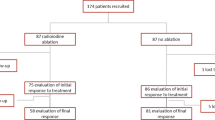
A total of 140 patients were enrolled in the study through October 2014–June 2015. Until February 2016, 132 patients completed the trial. 99 patients were re-examined under thyroid-stimulating hormone (TSH) stimulation 3–9 months after 131I therapy. For the low-dose and high-dose groups, the success rates of ablation were 52.7 % (29/55) and 59.1 % (26/44), respectively. The ablation results did not differ significantly between the two groups (P = 0.548). One hundred and thirty two patients were re-examined 2–9 months after 131I therapy. The low-dose group had an excellent response rate of ~80 % (53/66), an indeterminate response rate of ~20 % (13/66), and no cases with a biochemical incomplete response. The high-dose group had an excellent response rate of ~85 % (56/66), an indeterminate response rate of ~11 % (7/66), and a biochemical incomplete response rate of ~4 % (3/66). No significant differences in the therapeutic response were observed between the two groups (P = 0.087). Patients in stage N1b had a significantly lower success rate of ablation than those in stage N0 (P = 0.000). The success rate of ablation increased significantly with lower thyroglobulin (Tg) levels (P = 0.000). A pre-treatment Tg level was significantly associated with a higher excellent response rate (P = 0.002). Pre-treatment-stimulated Tg of 0.47 and 3.09 μg/L were identified as cut-off values for predicting the ablation result and therapeutic response, respectively. The incidences of adverse reactions were 18 % (12/66) and 39 % (26/66) in the low-dose and high-dose groups, respectively, and this difference between the two groups was significant (P = 0.007).
Conclusions
The result of thyroid remnant ablation and the response to therapy did not differ significantly between the two groups. The low-dose group had a lower incidence of adverse reactions than the high-dose group. N1b and pre-treatment-stimulated Tg were factors influencing the ablation result, whereas pre-treatment-stimulated Tg was a factor influencing the therapeutic response. Pre-treatment-stimulated Tg of 0.47 and 3.09 μg/L were identified as cut-off values for predicting the ablation result and therapeutic response, respectively. The study protocol was approved by the Clinical Trials and Biomedical Ethics Committee of our hospital and registered on the Chinese Clinical Trial Registry under the registration number ChiCTR-IOR-15006139.






Similar content being viewed by others
References
Stewart B, Wild CP. World cancer report 2014. World: 2015. http://publications.iarc.fr/Non-Series-Publications/World-Cancer-Reports/World-Cancer-Report-2014. Accessed 25 Mar 2016.
Sipos JA, Mazzaferri EL. Thyroid cancer epidemiology and prognostic variables. Clin Oncol. 2010;22(6):395–404.
Haugen BR, Alexander EK, Bible KC, Doherty GM, Mandel SJ, Nikiforov YE, et al. 2015 American Thyroid Association Management Guidelines for Adult Patients with Thyroid Nodules and Differentiated Thyroid Cancer: the American Thyroid Association Guidelines Task Force on Thyroid Nodules and Differentiated Thyroid Cancer. Thyroid. 2016;26(1):1–133.
Mäenpää HO, Heikkonen J, Vaalavirta L, Tenhunen M, Joensuu H. Low vs. high radioiodine activity to ablate the thyroid after thyroidectomy for cancer: a randomized study. PLoS One. 2008;3(4):e1885.
Mallick U, Harmer C, Yap B, Wadsley J, Clarke S, Moss L, et al. Ablation with low-dose radioiodine and thyrotropin alfa in thyroid cancer. N Engl J Med. 2012;366(18):1674–85.
Schlumberger M, Catargi B, Borget I, Deandreis D, Zerdoud S, Bridji B, et al. Strategies of radioiodine ablation in patients with low-risk thyroid cancer. N Engl J Med. 2012;366(18):1663–73.
Fallahi B, Beiki D, Takavar A, Fard-Esfahani A, Gilani KA, Saghari M, et al. Low versus high radioiodine dose in postoperative ablation of residual thyroid tissue in patients with differentiated thyroid carcinoma: a large randomized clinical trial. Nucl Med Commun. 2012;33(3):275–82.
Mazzaferri EL, Robbins RJ, Spencer CA, Braverman LE, Pacini F, Wartofsky L, et al. A consensus report of the role of serum thyroglobulin as a monitoring method for low-risk patients with papillary thyroid carcinoma. J Clin Endocrinol Metab. 2003;88(4):1433–41.
Schlumberger M, Berg G, Cohen O, Duntas L, Jamar F, Jarzab B, et al. Follow-up of low-risk patients with differentiated thyroid carcinoma: a European perspective. Eur J Endocrinol. 2004;150(2):105–12.
Robbins RJ, Chon JT, Fleisher M, Larson SM, Tuttle RM. Is the serum thyroglobulin response to recombinant human thyrotropin sufficient, by itself, to monitor for residual thyroid carcinoma? J Clin Endocrinol Metab. 2002;87(7):3242–7.
Pacini F, Capezzone M, Elisei R, Ceccarelli C, Taddei D, Pinchera A. Diagnostic 131-iodine whole-body scan may be avoided in thyroid cancer patients who have undetectable stimulated serum Tg levels after initial treatment. J Clin Endocrinol Metab. 2002;87(4):1499–501.
Jeon EJ, Jung ED. Diagnostic whole-body scan may not be necessary for intermediate-risk patients with differentiated thyroid cancer after low-dose (1.1 GBq) radioactive iodide ablation. Endocrinol Metab. 2014;29(1):33–9.
Tuttle RM, Tala H, Shah J, Leboeuf R, Ghossein R, Gonen M, et al. Estimating risk of recurrence in differentiated thyroid cancer after total thyroidectomy and radioactive iodine remnant ablation: using response to therapy variables to modify the initial risk estimates predicted by the new American Thyroid Association staging system. Thyroid. 2010;20(12):1341–9.
Vaisman F, Momesso D, Bulzico DA, Pessoa CH, Dias F, Corbo R, et al. Spontaneous remission in thyroid cancer patients after biochemical incomplete response to initial therapy. Clin Endocrinol. 2012;77(1):132–8.
Castagna MG, Maino F, Cipri C, Pessoa CH, Dias F, Corbo R, et al. Delayed risk stratification, to include the response to initial treatment (surgery and radioiodine ablation), has better outcome predictivity in differentiated thyroid cancer patients. Eur J Endocrinol. 2011;165(3):441–6.
Evans C, Tennant S, Perros P. Thyroglobulin in differentiated thyroid cancer. Clin Chim Acta. 2015;444:310–7.
Spencer C, Fatemi S, Singer P, Nicoloff J, Lopresti J, et al. Serum basal thyroglobulin measured by a second-generation assay correlates with the recombinant human thyrotropin-stimulated thyroglobulin response in patients treated for differentiated thyroid cancer. Thyroid. 2010;20(6):587–95.
Bachelot A, Cailleux AF, Klain M, Baudin E, Ricard M, Bellon N, et al. Relationship between tumor burden and serum thyroglobulin level in patients with papillary and follicular thyroid carcinoma. Thyroid. 2002;12(8):707–11.
Verburg FA, de Keizer B, Lips CJ, Zelissen PM, de Klerk JM, et al. Prognostic significance of successful ablation with radioiodine of differentiated thyroid cancer patients. Eur J Endocrinol. 2005;152(1):33–7.
Park HJ, Jeong GC, Kwon SY, Min JJ, Bom HS, Park KS, et al. Stimulated serum thyroglobulin level at the time of first dose of radioactive iodine therapy is the most predictive factor for therapeutic failure in patients with papillary thyroid carcinoma. Nuclear Med Moler Imaging. 2014;48(4):255–61.
Ha S, Oh SW, Kim YK, Koo DH, Jung YH, Yi KH, et al. Clinical outcome of remnant thyroid ablation with low dose radioiodine in korean patients with low to intermediate-risk thyroid cancer. J Korean Med Sci. 2015;30(7):876–81.
Van Nostrand D, Atkins F, Yeganeh F, Acio E, Bursaw R, Wartofsky L, et al. Dosimetrically determined doses of radioiodine for the treatment of metastatic thyroid carcinoma. Thyroid. 2002;12(2):121–34.
Luster M, Clarke SE, Dietlein M, Lassmann M, Lind P, Oyen WJ, et al. Guidelines for radioiodine therapy of differentiated thyroid cancer. Eur J Nucl Med Mol Imaging. 2008;35(10):1941–59.
Ceccarelli C, Bencivelli W, Morciano D, Pinchera A, Pacini F. 131I therapy for differentiated thyroid cancer leads to an earlier onset of menopause: results of a retrospective study. J Clin Endocrinol Metab. 2001;86(8):3512–5.
Vini L, Hyer S, Al-Saadi A, Pratt B, Harmer C. Prognosis for fertility and ovarian function after treatment with radioiodine for thyroid cancer. Postgrad Med J. 2002;78(916):92–3.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
No potential conflicts of interest were disclosed.
Additional information
Y. Qu and R. Huang are contributed equally to this work.
Rights and permissions
About this article
Cite this article
Qu, Y., Huang, R. & Li, L. Low- and high-dose radioiodine therapy for low-/intermediate-risk differentiated thyroid cancer: a preliminary clinical trial. Ann Nucl Med 31, 71–83 (2017). https://doi.org/10.1007/s12149-016-1133-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12149-016-1133-4