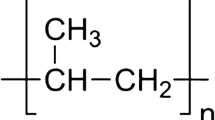Abstract
In this paper the possibility for authentication and differentiation of various styrene butadiene rubbers (SBRs) was investigated. Seven types of SBR were analyzed by multi-capillary column (MCC) ion mobility spectrometer (IMS) and their spectra compared. The analysis of volatile organic compounds (VOCs) releasing from the rubbers revealed the presence of characteristics signals, which can be assigned only to a specific material. Such “markers”, when defined for other polymer materials, can be used for their authentication. In the second part of the paper, the blend of epoxidized natural rubber and poly-3-hydroxybutyrate-co-4-hydroxybutyrate (ENR/P(3,4)HB) was subjected to different types of aging. MCC-IMS spectra of not aged, thermal, climatic and UV aged samples were collected and differences between the signals discussed. The study showed possibility of authentication of polymeric materials and processes. The paper is a some kind of introduction to the use of analytical properties and advantages of MCC-IMS technique in chemistry, technology and exploitation of polymer materials.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction to multi-capillary column – ion mobility spectrometry technique
Ion mobility spectrometry (IMS) is one of the most evolving and increasingly used techniques in many fields of science and industry. One of the main fields of its application are military, food processing and quality, process control or medicine. J. I. Baumbach presented a detailed overview of the literature on IMS in the International Journal on IMS from 1998 to 2007 [1]. Since 1998 to 2007 in databases like Web-Of-Science, SciFinder or Google Scholar using the keywords: “ion”, “mobility” and “spectrometry” one can find about 130,000 matching documents. Since 2008 to present, using the full sentence: “ion mobility spectrometry” one obtaines about 7000 publications in Web-Of-Science database, whereas before 2008 it was only 1326. It confirms on noticeably increasing interest and the amount of research with application of IMS technique.
Principles of analysis
Principles of Ion Mobility Spectrometry (IMS) are based on characterization of materials from the time of flight spectra of their ionized fragments, in an electric field and through a gas atmosphere. This definition includes various gas flow rates, pressure, type of gases, electric field strength and control and methods of samples’ ionization [2]. Ion mobility spectrometer consists of two main parts: a ionization chamber and a drift tube (drift region) (Fig. 1).
Scheme of ion mobility spectrometer [3]
Ionization chamber is the part of IMS where the formation of gas phase ions takes place. The ionization in analytical IMS is commonly realized in synthetic air atmosphere at ambient pressure. Under such conditions the amount of humidity and oxygen in the air are important parameters. Generally, applied ionization methods are make use of radioactive source (nickel, americium, tritium), corona discharge (CD), photodischarge lamps, lasers and various electrospray ion sources. Ionization techniques used mainly in laboratory or experimental fields: are radio-frequency (RF), flames, surface ionization and new sources like glow discharge and helium plasma [4]. Depending on the application particular techniques have advantages and limitations, for example electrospray is suitable mostly for liquid samples. In order to reach a proper level of sensitivity for analysis of some isomeric compounds, e.g. dihalogenated benzenes it is recommended to use a CD source [5].
In a ionization chamber a reservoir of ions, known as reactant ions, is generated. This chemical individuals originate in air at ambient pressure and depending on the reagent gas, are of positive polarity H+(H2O) n (positive reactant ion – RIP) or negative polarity \( {O}_2^{-}{\left({H}_2O\right)}_n \) (negative reactant ion – RIN) or both [6, 7]. The reaction of ions of positive polarity and molecules of analyte relies on collisions with hydrated proton with the creation of cluster ion, which is stabilized through the dislocation of adducted water to give a product ion - a protonated monomer as Eq. 1.1
- 1:
-
analyte molecule + reactant ion
- 2:
-
cluster ion
- 3:
-
protonated monomer + water
If the concentration of analyte increases, it can lead to formation of the second product ion, in which another molecule attaches to the protonated monomer, displacing a water molecule and yielding a proton-bound dimer M2H+ − (H2O)n − x as Eq. 1.2:
- 1:
-
protonated monomer + analyte molecule
- 2:
-
proton bound dimer + water
Subsequent formation of proton-bound trimers, tetramers etc. may occur in the gas saturated region of the ion source. This structures have short lifetimes and are seldom observed in ion mobility spectra.
In analogy to the formation of the positive ions, reaction of the molecule and an oxygen anion takes place according to the Eq. 1.3:
- 1:
-
analyte molecule + negative reactant ion
- 2:
-
cluster ion
- 3:
-
product ion + water
Depending on the chemical structure of analyte some of compounds are susceptible to reactions with ions of the appropriate polarity. This compounds property is also related to proton affinity which affects selectivity in mobility spectra. A result of high collision rate and relatively long presence in an ionization chamber makes IMS a trace detector with some control of selectivity concerning ion formation and ion mobility [2].
Between a ionization chamber and drift tube a shutter grid is located which passes a batch of ions into the drift tube. In the drift region, ions moves through purified air at ambient pressure in a voltage gradient or electric field (E in V/cm). Drift velocities for ions are calculated from time (t d in ms) which swarm need to cross the distance (d in cm) between the ion shutter grid and a detector. t d is called drift time of ions:
Values of drift velocity and electric field are needed to calculate the mobility coefficient (K) which gives a data normalization in cm2/Vs:
In the drift region the velocity of ions is influenced by temperature (T) and pressure (p) and K is commonly normalized to standard values of T and P, giving the reduced mobility coefficient K o :
To make IMS more effective for analysis of volatile organic compounds, the IMS instrumentation is often coupled with a multi capillary (MCC) or gas chromatography column (GC). Sample is pre-separated on MCC and then an analyte goes to IMS directly. This combination gives possibility to characterize every type of ions by an additional parameter, which is retention time (t r ). As a consequence of this connection, all types of ions are described by two characteristic values - t d and t r and additionally by the intensity of signal (I in mV) which depends on the amount of ions.
Examples of application
The idea of application of MCC-IMS in rubber chemistry and technology comes from the current trends of its application for solving scientific and industrial problems. A significant increase in interest of this technique, for example in medicine and other fields, gives a chance to use it for exploring specific ideas in various fields of application. One of the most common use of MCC-IMS is looking for specific compounds, markers and chemical species which can be responsible for a quality of some materials or products. Moreover, dangerous substances, toxic, irritant or harmful, which are unexpected and may adversely affect the environment or technological process can be easily detected by means of presented technique. It should be remembered that MCC-IMS analysis offers many possibilities to use. Generally, the technique is considered to be one of the relatively new analytical methods, also in rubber science and engineering.
J. Stach et al. in Ion Mobility Spectrometry – Basic Elements and Applications [8] divided the application of IMS or IMS coupled with different chromatographic methods for presepration on four groups: chemical warfare agents (CWA), explosives, drugs and generally - working place monitoring [9]. Detection of CWA and military application of IMS were the first developments. There was a simple, not hyphenated IMS modulus, focused on the detection of one or more warfare agents. One of the first was CAM analyzer manufactured by the Graseby Dynamics Company from the United Kingdom [9]. Popularization and working on the improvement of IMS had lasted still both before and after the market debut.
Nowadays, the application of MCC-IMS croses previous boundaries. This instruments are used much wider and in many interdisciplinary fields. Beginning from the medical or pharmaceutical applications, there is a plenty of potential uses, which include health and safety monitoring, biomedical markers analysis or cleaning verification [10, 11]. Also, in the field of foodomics many applications were observed – e.g. assessment of food freshness, detection of traces of harmful chemicals in food and olive oil or oxidation process in peanuts [12,13,14].
The idea for this study bases on finding markers (like in medical or foodomics applications), specific fingerprints which are characteristic for specific type of processes and compositions of polymers and polymer materials. There is a plenty of advantages for using IMS in general: low price system, rapidity of measurements, high selectivity and possibility to control processes on-line, justifying promotion of its application also in various aspects of rubber chemistry and technology. Motivation to study SBR comes from the fact that it is one of the most popular materials used in rubber industry: tires, hoses, seals, conveyor belts, rubber covers and many others. Possibility of authentication materials which consists mentioned SBR rubbers is important due to environmental protection, health and safety or other aspects associated with the production of the rubber goods. In addition, studying raw rubber matrix allows for further analysis of more complex systems which consists of another additives like: curing agents, fillers, antioxidants, oils, etc.
Experimental
Materials and measurement methodology
The first study involves seven types of emulsion polymerization SBR (styrene-butadiene rubber): KER 1500 (Synthos Rubber, Poland), INTOL 1509 (Polimeri Europa), Europrene 1502 (Versalis, Italy), KER 1502 (Synthos Rubber, Poland), Sprintan 4630 (Styron/Trinseo, Germany) and two prototype samples of solution polymerization S-SBRs, received from industry: S-SBR, 25 s containing TDAE oil and S-SBR 30s containing HN oil, which characteristic remains so far confidential.
The second experiment is devoted to vulcanizates of the blend of epoxidized natural rubber and poly-3-hydroxybutyrate-co-4-hydroxybutyrate (ENR/P(3,4)HB), subjected to thermal, climatic and UV aging. ENR of 50% of epoxidation rate was obtained from Kumpulan Guthrie Berhad (Malesia), whereas P(3,4)HB resin from SigmaChem (China).
Aging methodology
Ageing characteristics were determined according to PN-82/C-04216 standard. Samples were subjected to the action of air at elevated temperature (353 K) for 7 days in a laboratory oven with thermo-circulation. UV ageing was performed by means of an UV 2000 apparatus from Atlas. The measurement lasted for 120 h and consisted of two alternately repeating segments with the following parameters: daily segment (radiation intensity 0.7 W/m2, temperature 60 °C, duration 8 h), night segment (no UV radiation, temperature 50 °C, duration 4 h). Climatic ageing was carried out using a Weather-Ometer (Atlas; Ci 4000). The test was based on two variable segments simulating day and night conditions, and the samples were subjected to two different cycles: daily cycle (radiation intensity 0.4 W/m2, temperature 60 °C, duration 240 min, humidity 80%, rain water on), night cycle (no radiation, temperature 50 °C, humidity 60%, duration 120 min).
GC-IMS and sample introduction systems
The experimental system (Fig. 2) consists of a mass flow controller (MFC1) from Aalborg: flow range: 0–250 ml/min with the error of 1%. The “analyte” on the scheme means a chamber for a sample – 100 ml glass flask with a plastic lid, which is connected to 0.3 cm external diameter PTFE hoses. The glass flask is submerged in oil bath equipped with a magnetic stirrer and a heater, what gives possibility to maintain constant temperature of the sample. Output cable is connected into a GC-IMS unit Version 1.0 from GAS Dortmund (Germany). The system consist of the IMS coupled to a gas chromatographic column (MCC) (detailed specification given in Table 1). The drift gas for the IMS is supplied using an electronic pressure control unit (EPC1). The carrier gas for the column is supplied using a second electronic pressure control unit (EPC2). Both gases (carrier and drift gas) leave the device at gas out. IMS, MCC and 6-Port Valve with sample loop are heated (T1, T2 and T3). The sample is carried via the heated 6-Port Valve (T3) to the MCC. In the default position of the 6-point valve the carrier gas permanently flushes the multi capillary column. The sample gas is pumped through the loop by the pump P (“Fill loop” phase). Ends of all PTFE hoses are fixed with standard SWAGELOK connectors. The gas used for all analyses (carrier gas and drift gas) is a synthetic air of clinical purity from Linde Group (Poland). The exit of a gas bottle has a filter mounted. The MCC-IMS works with the “positive charge” mode, so only cations are detected.
Measurement methodology
All SBR rubbers were prepared and measured in the same way and under the same conditions: 1 g samples were chopped into smaller pieces and then put into a 4 ml chromatographic glass vial. Then the vials were inserted into a preheated measurement chamber (100 ml glass flask designated as “analyte” on Fig. 2) and after closing the chamber were flushed with a clean synthetic air. After that, samples were heated up to a defined specific temperature and kept during the proposed time, before measurement. The gas phase collected in 100 ml glass flask was then injected into MCC-IMS in time of injection to sample loop (Table 2). The injection was effected by the forced flow of synthetic air. For each sample, the measurement was repeated 3 times. For second experiment, involving ENR/P(3,4)HB, preparation of samples was similar to the method used for SBRs, but the temperatures and time of collecting volatiles were different. The conditions for both experiments are presented in Table 2. In this case, the calculations of the real concentration of signals, the calibration of reduced mobility coefficient and quantitative analysis was not needed. Values of retention times (t r in s) and drift time (ms) or relative RIP position were determined by the software coupled with the device.
Results and discussion
Analysis of SBR rubbers
First study and spectra of the measured SBR samples demonstrated the complexity of the gas phase. SBR is produced by copolymerization of butadiene with styrene in the approximate proportion of 3:1 by weight. Also an initiators, catalyst and stabilizers are employed. Manufacturers use various types of chemistries in case of different types of SBR e.g. rosy and fatty acids soaps and “staining” or “non-staining” antioxidants. Specific type of chemistries are guarded by trade secrets. For instance, chemically similar rubber like Europrene 1502 and KER 1502, but produced by different manufactures, produce different spectra of gas phase. Result of that phenomenon can be a possibility of designation of a “finger print” for each material, which can be detected under thermal conditions (close to rubber processing). First, spectra of all SBR studied are presented in Fig. 3. On the first look a lot of different signals and different intensities can be distinguished.
Next step for searching differences between the MCC-IMS spectra (Fig. 3) was to analyze the signals present and to create a map of them. Each signal is described by two values – retention time and drift time. For signals with long tails, the retention time was the initial part of a peak. The graphical mapping was created by software, which allows to capture the entire range of a given signal. Clipping of signals are arranged in the sequence of areas for each measurement. Established group of areas, consisting of all selected signals, can be saved and exported to external plug. External program can compare the loaded spectrum in relation to these areas, registering the presence of changes to signal intensity. For the generation of the clippings the IMS signal intensities in selected areas are extracted and plotted in a 25 × 25 px box, whatever the original area size or area shape is. As a result of this comparison a matrix has been created. An overall map created after analyzing each spectrum separately has been compiled in one matrix (Fig. 4). The most specific and intensive signals were selected for the matrix. On “x” axis there is a numbers of signals which were picked for all type of rubbers, whereas on “y” axis the type of rubber. Precise interpretation of this matrix takes place when we took any signal and look for him perpendicularly. For 37 chosen signals, the specific peaks can be assigned to only one type of rubber (Fig. 5). Both the graphic and relevant values in the matrix (Table 2) demonstrate and confirm on this finding. Also, the “fingerprints” for each rubber were found. All signals were additionally compared with blank measurement spectra of gas introduction system without sample called on Fig. 4 “Dilution system + vial”. The number of compounds which can hide themselves behind peaks is extensive. Processes in which SBR rubbers participate was studied by other techniques like FTIR, DSC [15] and chromatographic methods coupled with mass spectrometry [16] in the case of more complex compounds, which contain SBR in their composition. Rubber processing occurs at elevated temperature involving oxygen. The main products resulting from thermal decompositions, free radical degradation, scission or thermal oxidation processes [15, 17]. Possible examples of chemical compounds released from SBR rubbers during their processing are benzaldehyde, α-methylstyrene, 4-phenylcyclohexene, 4-vinylcyclohexene, cyclohexene, dimethylstyrene and others [18]. Also, the groups of chemicals on different stages of the mentioned above thermal or thermal-oxidation processes as saturated ketones, aldehydes, esters, alcohols and others oxides have been proposed [15, 17, 19]. For qualitative analysis in this case, more experiments are needed (Table 3).
Studying aging of ENR/P(3,4)HB system
The idea behind the second study is the possibility to detect characteristic processes materials are involved in, on the example of aging of polymer materials. The results are not so clear as in the first study but differences between three types of ageing are obvious. Carrying out the experiments one can draw attention to the following aspects. First of all, the temperature of collecting volatiles is a very important parameter. Secondly, the temperature of collecting volatiles cannot be too high, due the possibility of increasing degradation rate for some of materials. Increase of temperature made new signals appeared and increased volatiles concentration (intensity of signals). Moreover, time of collecting volatiles in this case had to be selected in terms of the speed of the analysis itself. Equilibrium time was not needed because is the analysis of not a quantitative character.
In Fig. 6 four spectra of ENR/P(3,4)HB compound are presented – not aged sample and after subjection to three different types of aging. On the first look the biggest differences are between: not aged/thermal and climatic/UV aged spectra. The spectrum of not aged sample subjected to heating can simulate to some extent the processes of thermal-oxidative aging. This phenomenon can explain the similarity between spectra of not aged and thermal aged sample (Figs. 1, 2, and 6). Spectra number 3 and 4 in Fig. 6, representing the effect of climatic and UV aging of the samples respectively, consists additionally of the products of radiation (for UV daily segment - 0,4 W/m2 and for UV daily segment - 0,7 W/m2). Moreover, climatic ageing is conducted in increased humidity (80% for daily and 60% for night segment) and in the presence of water. Therefore, higher amounts of volatile organic compounds, are expected, due to possible hydrolysis reactions, destructive radiation, as well as thermal degradation and oxidation processes. In Fig. 7 the selected areas of each signals, which can be distinguished, are observed. Each of 23 signals have been compiled in the signal matrix, as presented in Fig. 8. Analysis of the matrix makes possible to draw some conclusions. For climatic and UV aging, about 13 more signals are present than for not aged and thermal aged sample. The number of aging indicative signals in thespectra of irradiated samples is similar. Nevertheless, small differences between intensities and resolutions of the signals can be observed. “Signal 18” seems to be the original signal for UV aging (the marker of UV aging). As for the previous studies on SBRs, there are many volatile organic compounds being represented and covered under one peak. Based on analysis of MCC-IMS spectra, oxidation processes of ENR and poor aging resistance of rubber can be explained by ring-opening reactions of its epoxide groups [20, 21]. Moreover, for unoxidized samples of ENR chemistries like tetrahydrofuran ring appears as a secondary by-product of epoxide ring-opening reaction, which occurs during the preparation of ENR. Also glycol and hydroxyl formate appear from acid catalyzed reactions on epoxide ring during ENR preparation [22]. After oxidation process, hydroperoxide, carboxylic acids, alcohols, esters and other carbonyl compounds appear – reaction products occurred in the double bound region and epoxide ring-opening [21]. For P(3,4)HB as biodegradable material the most scientific reports refer to polymer biodegradation or bacterial aging. However, the common processes during UV-photodegradation of that type of materials are chain scission and crosslinking reactions [23].
Conclusions
The results obtained in this work showed enormous potential of MCC-IMS technique for analysis of polymer materials, processes and chemical phenomena related to them. The first study showed that the MCC-IMS can be used as a authentication tool, for finding the characteristic signals enabling unambiguous identification of specific types even of very similar materials. Moreover, the second study demonstrated that the MCC-IMS can distinguish between different types of aging process exactly on the same sample. The results on application of GC-IMS in rubber chemistry and technology presented in this work have pioneering character and have to be further developed. Nevertheless the technique deserves interest from the point of view of its application for a quick qualitative and quantitative analysis of materials and accompanied modification, being the result of their processing or exploitation. Possibility of creating database of pure chemistries for the processes presented in this study is the next step. This may lead to a better understanding of the processes involved in the processing and exploitation of polymer materials. Also the possibility for analysis of reaction kinetics as well as compiling GC-IMS with other analyses, like TG or GC-MS can move polymer science towards better understanding of phenomena related to these materials.
References
Baumbach JI (2008) Ion mobility spectrometry in scientific literature and in the International Journal for Ion Mobility Spectrometry (1998–2007). Int J Ion Mobil Spectrom 11:3–11
Eiceman GA, Karpas Z, Hill HH Jr (2014) Ion mobility spectrometry. CRC Press Taylor & Francis Group, Boca Raton ISBN: 978-1-4398-5997-1
Hauschild AC, Kopczynski D, D’Addario M, Baumbach JI, Rahmann S, Baumbach J (2013) Peak detection method evaluation for ion mobility spectrometry by using machine learning approaches. Meta 3(2):277–293
Rodriguez J, Lopez-Vidal S (2009) Sampling methods for ion mobility spectrometers: sampling, preconcentration and ionization. Project No. 217925, LOTUS TR-09-007
Borsdorf H, Nazarov EG, Eiceman GA (2004) Atmospheric pressure ionization and gas phase ion mobility studies of isomeric dihalogenated benzenes using different ionization techniques. Int J Mass Spectrom 232:117–126
Siu KWM, Aue WA (1987) 63Ni β range and backscattering in confined geometries. Can J Chem 65(5):1012–1024
Arshadi M, Kebarle P (1970) Hydration of OH− and O2− in the gas phase. Comparative salvation of OH− by water and the hydrogen halides effects of acidity. J Phys Chem 74(7):1483–1485
Stach J, Baumbach JI (n.d.) Ion mobility spectrometry – basic elements and applications. Int J Ion Mobil Spectrom 5:1–21
Blyth DA (1983) A vapour monitor for detection an contamination control. Proceedings of the international symposium on protection against CWA, Stockholm, Sweden, 6–9 June, pp 65–69
Szymanska E, Tinnevelta GH, Brodrickc E, Williamsc M, Daviesc AN, van Manene HJ, Buydens LMC (2016) Increasing conclusiveness of clinical breath analysis by improved baseline correction of multi capillary column – ion mobility spectrometry (MCC-IMS) data. J Pharm Biomed Anal 127:170–175
O’Donnell RM, Sun X, Harrington PB (2008) Pharmaceutical applications of ion mobility spectrometry. Trends Anal Chem 27:1
Karpas Z (2013) Applications of ion mobility spectrometry (IMS) in the field of foodomics. Food Res Int 54:1146–1151
Tzschoppe M, Haase H, Hoohnisch M, Jaros D, Rohm H (2016) Using ion mobility spectrometry for screening the autoxidation of peanuts. Food Control 64:17–21
Camara M, Gharbi N, Lenouvel A, Behr M, Guignard C, Orlewski P, Evers D (2013) Detection and quantification of natural contaminants of wine by gas chromatography−differential ion mobility spectrometry (GC-DMS). Food Chem 61:1036–1043
Guo L, Huang G, Zheng J, Li G (2014) Thermal oxidative degradation of styrene-butadiene rubber (SBR) studied by 2D correlation analysis and kinetic analysis. J Therm Anal Calorim 115:647–657
Gągol M, Boczkaj G, Haponiuk J, Formela K (2015) Investigation of volatile low molecular weight compounds formed during continuous reclaiming of ground tire rubber. Polym Degrad Stab 119:113–120
Vernaez O, Dagreou S, Grassl B, Müller AJ (2015) Degradation of styrene butadiene rubber (SBR) in anaerobic conditions. Polym Degrad Stab 111:159–168
Baumann W, Ismeier M (1998) Kautschuk and gummi – daten und fakten zum umweltschutz band 1/2. pp 247–248
Choi S-S (2002) Characteristics of the pyrolysis patterns of styrene-butadiene rubbers with differing microstructures. J Anal Appl Pyrolysis 62:319–330
Roy S, Gupta BR, Chaki TK (1993) Studies on the aging behavior of gum epoxidized natural rubber. Kautsch Gummi Kunstst 46(4):293
Gelling IR, Morrison NJ (1985) Sulfur vulcanization and oxidative aging of epoxidized natural rubber. Rubber Chem Technol 58(2):243
Ng SC, Gan LH (1981) Reaction of natural rubber latex with performic acid. Eur Polym J 17:1073–1077
Jungseop L, Jonghoon K (2016) UV-photodegradation of poly(3-hydroxybutyrate-co-3-hydroxyhexanoate) (PHB-HHx). Macromol Res 24(1):9–13
Author information
Authors and Affiliations
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Pietrzak, D., Bieliński, D.M. Application of multi-capillary column – ion mobility spectrometry (MCC-IMS) in rubber chemistry and technology. Int. J. Ion Mobil. Spec. 21, 1–9 (2018). https://doi.org/10.1007/s12127-018-0229-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12127-018-0229-z