Abstract
Classification of tumors of the head and neck has evolved in recent decades including a widespread application of molecular testing in tumors of the sinonasal tract, salivary glands, and soft tissues with a predilection for the head and neck. The availability of new molecular techniques has allowed for the definition of multiple novel tumor types unique to head and neck sites. Moreover, an expanding spectrum of immunohistochemical markers specific to genetic alterations facilitates rapid identification of diagnostic molecular abnormalities. As such, it is currently possible for head and neck pathologists to benefit from a molecularly defined tumor classification while making diagnoses that are still based largely on histopathology and immunohistochemistry. This review covers the principal molecular alterations in sinonasal malignancies, such as alterations in DEK, AFF2, NUTM1, IDH1-2, and SWI/SNF genes in particular, that are important from a practical standpoint for diagnosis, prognosis, and prediction of response to treatment.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Classification of head and neck neoplasms has improved in recent decades with the widespread application of molecular testing. Not only has molecular testing allowed for the definition of multiple novel tumor types unique to the head and neck, but it has also facilitated the recognition of ubiquitous tumors that commonly involve the head and neck. Molecular testing has illuminated the pathogenesis of well-established but previously enigmatic entities and clarified the relationships between various neoplasms. The current 5th edition of the World Health Organization (WHO) Classification of Head and Neck Tumours relies heavily on molecular data to support the inclusion of several new tumor entities and their subtypes and to provide detailed prognostic and pathogenetic information [1]. However, it must be emphasized that molecular testing alone is not sufficient to make the correct diagnosis in head and neck pathology. In fact, on top of the existing histologic morphologic entities, now, molecular biology provides additional information that helps in fine-tuning subtypes and boundaries that previously were not crystal clear. Moreover, the availability of an expanding spectrum of immunohistochemical markers facilitates the rapid identification of useful diagnostic molecular features. As such, it is currently possible for head and neck pathologists to benefit from a molecularly defined classification while still making diagnoses based largely on histopathology and immunohistochemistry.
In recent years, considerable progress in sinonasal tumor taxonomy has taken place with the discovery of tumor type-specific fusion oncogenes generated by chromosomal translocations (such as DEK::AFF2 and NUTM1 gene fusions), as well as recognition of inactivated tumor suppressor genes, such as SWI/SNF deficiency (detectable by immunohistochemistry), unique to specific tumor types. This review covers the principal molecular alterations that are important in sinonasal malignant neoplasms from a practical standpoint for diagnosis, prognosis, and prediction of response to treatment. Currently, new therapeutic approaches for sinonasal malignant tumors are urgently needed. For most histological subtypes, surgery is considered the gold standard of treatment when feasible, frequently complemented by adjuvant radiotherapy [2]. Survival has not improved significantly in recent years with this traditional approach, and ongoing efforts focus now on combining existing treatment strategies such as induction chemotherapy with preceding innovative radiation techniques. However, the results remain often disappointing [3]. We aim to contribute to the appreciation of the intersecting roles of molecular testing and more conventional diagnostic modalities in sinonasal tumors.
Sinonasal epithelial malignant neoplasms
The sinonasal tract, comprising the nasal cavity, the paranasal sinuses, and the anterior skull base, is an anatomic region characterized by a broad spectrum of tumors that exhibit a significant morphological diversity of molecularly defined entities (Table 1). The recent 5th edition of the WHO Classification of Head and Neck Tumours includes new entities in which molecular genetics have an important diagnostic role, including HPV-related multiphenotypic sinonasal carcinoma, SWI/SNF-deficient sinonasal carcinoma and adenocarcinoma [1], and a subset of emerging entities including the IDH-mutated malignancies classified in the category of sinonasal undifferentiated carcinoma (SNUC) [1]. Molecular genetics also plays a diagnostic role in the context of hereditary syndromes manifested in the sinonasal tract.
Non-keratinizing squamous cell carcinoma including those with DEK::AFF2 gene fusion
Sinonasal non-keratinizing squamous cell carcinoma (NKSCC) comprises a heterogeneous category of neoplasms of different etiological and molecular pathogenesis. It became increasingly evident that NKSCC as a merely descriptive diagnosis is not valid anymore. Notably, a subset of these tumors is driven by transcriptionally active human papilloma virus (HPV), most commonly type 16, in 36–58% of patients with this diagnosis. While routine HPV testing is not recommended in sinonasal NKSCC, it can occasionally be helpful for diagnostic purposes. If HPV testing is carried out, HPV-specific tests such as in-situ hybridization or PCR must be used, as p16 immunohistochemistry has poor specificity in sinonasal tumors [1]. More recently, it has been found that more than half of the NKSCCs that are not associated with HPV reveal a recurrent translocation DEK::AFF2 [1]. These new developments point to the need for adopting a “diagnosis by exclusion” strategy for “NKSCC NOS” after the exclusion of these genetically defined subtypes.
DEK::AFF2 carcinoma
DEK::AFF2 carcinoma is currently classified as an emerging entity under the category of non-HPV-associated NKSCC, localized especially in the sinonasal tract; although, a few cases have been reported arising in the middle ear, temporal bone, orbita, and lung [4, 5]. Despite bland-looking morphology, this tumor behaves in an aggressive fashion with a high risk of local recurrence, metastatic nodal dissemination, and distant spread [6].
Histologically, most cases demonstrate a complex exophytic and endophytic growth of basaloid to transitional cells [7, 8]. Where present, the appearance of papillary fronds ranges from delicate to broad [8]. The tumor cells also grow into the underlying stroma forming anastomosing lobules, ribbons, and occasional nests and cords. The invasive pattern tends to be broad-based and pushing, but it may reveal a discohesive pattern of invasion with numerous small irregular nests widely infiltrating into the bone [7]. Nuclear palisading is frequently seen at the periphery of tumor lobules (Fig. 1A). Intraepithelial cell discohesion may result in pseudopapillary formation and stellate reticulum-like appearance in the center of tumor sheets. Typically, tumor cells possess bland-looking, monotonous, round to oval-shaped nuclei with fine to vesicular chromatin, prominent nucleoli, amphophilic to eosinophilic cytoplasm, and indistinct cell borders. The mitotic counts are usually low but a high mitotic index is also seen in a few cases [7, 9]. Tumor necrosis and apoptotic bodies are noted in some cases. Most tumors are densely infiltrated by abundant neutrophils in both the epithelium and the stroma (Fig. 1B) [7,8,9]. Microabscess formation can occur [9].
DEK::AFF2 carcinoma. Nuclear palisading is frequently seen at the periphery of tumor lobules (A). Most tumors are densely infiltrated by abundant neutrophils (inset) in both the epithelium and the stroma (B). All DEK::AFF2 carcinomas are diffusely positive for p63 and p40 (C) DEK::AFF2 carcinomas were successfully immunostained for AFF2 protein, and all cases showed positive nuclear expression (D)
All DEK::AFF2 carcinomas are diffusely positive for p63 and p40 (Fig. 1C) [5]. They are also positive for cytokeratins, including AE1/AE3 and CK5/6. DEK::AFF2 carcinomas have been successfully immunostained for AFF2 protein, and all cases showed positive nuclear expression (Fig. 1D) [10]. Accordingly, AFF2 immunohistochemistry represents an emerging highly sensitive and specific ancillary marker that distinguishes DEK::AFF2 carcinoma from other sinonasal tumors with overlapping morphological features, and it may also be useful in decalcified specimens [10]. The original case report on the entity describes a DEK::AFF2 carcinoma with an excellent response to immune checkpoint inhibitors (ICI)—anti-PD-L1—which is related to DEK::AFF2 neoantigen-specific T-cell response during tumor regression [4].
Sinonasal NUT Carcinoma
NUT Carcinoma is a highly aggressive, mostly lethal malignancy with monotonous poorly differentiated morphology. NUT carcinoma (formerly termed NUT midline carcinoma) has a predilection for mediastinum (approximately 50% of cases) and head and neck, particularly the sinonasal tract but also elsewhere, for example, the larynx [11]. Histologically, NUT carcinoma is a very poorly differentiated malignancy that grows as nests and sheets of tumor cells. NUT carcinoma is a highly infiltrative and cytologically high-grade malignancy with numerous mitotic figures and frequent tumor necrosis. A clue to diagnosis is the fact that despite the tumor being clearly high-grade, tumor nuclei lack significant pleomorphism that is typically seen in high-grade carcinomas. In contrast, the nuclei are relatively uniform and monotonous (Fig. 2A). In some NUT carcinomas, overt squamous differentiation is seen in the form of either ‘‘abrupt” keratinization, i.e., undifferentiated tumor cells are often seen immediately next to highly differentiated keratin pearls or as ‘‘abrupt’’ squamoid cells aggregates with copious clear cytoplasm within the undifferentiated basaloid cell aggregates (Fig. 2B). This overt squamous differentiation is seen in no more than 43% of cases [12].
NUT carcinoma. Nuclei are relatively uniform and monotonous (A). In some NUT carcinomas, abrupt keratinization is seen; undifferentiated tumor cells are present immediately next to highly differentiated keratin pearls (B). The tumor cells are strongly positive for cytokeratin CK5/6 (C). The monoclonal NUT antibody is highly specific for NUT carcinoma (D)
NUT carcinoma is considered to be a part of the spectrum of squamous cell carcinomas, which is supported by positivity for cytokeratin, CK5/6 in particular (Fig. 2C), and p63, while p40 is less reliably positive. The monoclonal NUT antibody is highly specific for NUT carcinoma (Fig. 2D) [13]. NUTM1-rearranged tumors such as skin adnexal poroid neoplasms and CIC::NUTM1 sarcoma are positive for NUT antibody by immunohistochemistry, but these tumors are almost never sinonasal [14, 15]. Limited focal expression of the wildtype NUT protein can be seen rarely in some conventional squamous cell carcinomas, but the staining is weak and focal, being present in < 20% of neoplastic cells. The typical punctate pattern of positivity is limited to NUT carcinoma, and it is seen in > 70% of cells and is often uniformly positive in all tumor cells.
NUT carcinoma is genetically characterized by a rearrangement of the NUTM1 gene (Nuclear Protein in Testis) on chromosome 15q14 [16]. The NUT gene is physiologically expressed in mature spermatogonia. The NUT-signaling molecule binds to histone acetyltransferase (HAT) p300 and activates histone acetylation. The most common fusion partners of NUT are genes involved in transcription and chromosome regulation belonging to the BET family (BRD2, BRD3, BRD4, and BRDT) [17]. In 75% of cases, the fusion partner of NUTM1 is the BRD4 gene (in 19p13.1), followed by BRD3 (in 9q34.2) in 15% of cases [18]. The NUT::BRD3/4 fusion oncoprotein functions by blocking cell differentiation and promoting uncontrolled cell growth [19]. In a subset of cases, NUT is fused with non-BRD genes. In 6% of the cases, the fusion involves the NSD3 gene (in 8p11.23), which codes for an oncoprotein required for differentiation and regulation of cell proliferation [20]. In 2% of the cases, genes for zinc finger-containing proteins, (ZNF532 on 18q21.32 or ZNF592 on 15q25.3) are involved in NUT fusion [21].
Until recently, there was no known effective treatment for NUT carcinoma which has a median survival of 6.7 months, explaining the name “ticket to heaven” for this aggressive entity [22]. Surgery and radiotherapy form the gold standard, and they may prolong progression-free survival and overall survival (OS); recently, somewhat better results were seen using an induction chemotherapy strategy [23]. In a recent study of 12 patients with sinonasal NUT carcinoma treated at MD Anderson Cancer Center, the median OS was 14.6 months. Patients with maxillary sinus tumors were 91% more likely to survive (hazard ratio [HR], 0.094; 95% confidence interval [CI], 0.011–0.78, p = 0.011). Patients with higher-stage disease stage had worse OS (stage IVb-c no 2-year survivors, vs. stage III-Iva 60% 2-year survival, p = 0.05). All three patients who were alive with no evidence of disease received induction chemotherapy [24]. The first targeted drugs for NUT carcinoma were histone deacetylase inhibitors (HDACi) (vorinostat) and BET inhibitors (BETi), which inhibit tumor cell growth and induce cell differentiation [25]. BETi (JQ1) molecules mimic acetylated histones and competitively inhibit the tethering of BRD3/4 to acetylated chromatin. In addition, BETi directly targets the NUT fusion protein [19]. Whether such targeting in NUT carcinoma results in a clinical response has not been investigated yet. Furthermore, no BETi has been granted FDA approval to date [26].
SWI/SNF complex deficient sinonasal carcinoma and other malignancies
The chromatin remodeling Switch/Sucrose non-fermentable complex (SWI/SNF) is a pleomorphic complex of over 20 tumor suppressors that communicate with transcription factors at the promotor site, mobilize nucleosomes, and modulate chromatin structure [27]. These genes are involved in cell differentiation and proliferation. There are four different subtypes of SWI/SNF complex deficient sinonasal/base of skull malignancies, including SMARCB1-deficient sinonasal carcinoma, SMARCB1-deficient sinonasal adenocarcinoma, SMARCA4-deficient sinonasal carcinoma, and a subset of SMARCA4-deficient teratocarcinosarcomas. Poorly differentiated chordomas are also SMARCB1-deficient, and they may rarely involve the sinonasal tract by extension from the skull base.
SMARCB1-deficient sinonasal carcinoma
SMARCB1 deficiency in a subset of poorly or undifferentiated sinonasal carcinoma was first recognized in 2014 by 2 independent groups [28, 29] followed by a few additional case series, the largest multi-institutional series comprised 39 patients [30]. SMARCB1-deficient sinonasal carcinoma is defined by a lack of features of any other defined sinonasal carcinoma type, complete loss of SMARCB1 expression, and absence of morphologic squamous or glandular differentiation. Most cases show basaloid cell morphology but squamous features and keratinization are absent. Around 30% of cases, however, show eosinophilic cells with rhabdoid and/or plasmacytoid features. The tumor cells usually display large solid nests and sheets. Immunohistochemically, the neoplastic cells of SMARCB1-deficient sinonasal carcinoma are uniformly pan-cytokeratin positive, focally positive for p63/p40, and negative for NUT and p16 [31].
SMARCB1-deficient sinonasal adenocarcinoma
This is a rare SWI/SNF-deficient malignancy defined by the presence of unequivocal glandular differentiation and/or by the presence of other features of adenocarcinoma [32]. Tumor histomorphology is predominantly solid, with trabecular and alveolar growth patterns. The tumor cells are large with eosinophilic, oncocytoid, plasmacytoid, and/or rhabdoid appearance (Fig. 3A). SMARCB1-deficient sinonasal adenocarcinoma demonstrates varying proportions of glandular formations, including alveolar/acinar structures with abortive microglandular differentiation, trabecular, and solid/cribriform/insular patterns. Areas with yolk sac tumor-like differentiation including Schiller–Duval body-like structures are often found (Fig. 3B). Immunohistochemical markers for yolk sac tumor (SALL4 or glypican-3) are often seen (Fig. 3C), corresponding to their yolk sac tumor-like histologies. SMARCB1-deficient sinonasal adenocarcinomas lack the basaloid morphology seen in 60–70% of SMARCB1-deficient sinonasal carcinomas and instead display either clear-cut gland formation, cribriform patterns, or mucin production. Some of them are reminiscent of high-grade non-intestinal type adenocarcinoma while others may closely mimic myoepithelial carcinomas. The yolk sac tumor-like pattern is limited to the adenocarcinoma subgroup. While focal p63 and/ or CK5/6 immunopositivity can be seen, they lack the diffuse uniform pattern of squamous cell type, and yolk sac markers are frequently expressed. However, tumors with transitional features between the two types are seen indicating a morphological spectrum.
SMARCB1 deficient adenocarcinoma of the nasal cavity. Tumor cells are large (inset) with eosinophilic, oncocytoid, plasmacytoid, and/or rhabdoid appearance (A). Areas with yolk sac tumor-like differentiation including Schiller–Duval body-like structures are found (B). Immunohistochemical staining for yolk sac tumor marker SALL4 is often positive (C). Tumor cells are devoid of INI1 immunostaining (D)
SMARCA4-deficient sinonasal carcinoma
Since the first detailed description by Agaimy and Weichert [33], no more than 22 cases of SMARCA4-deficient sinonasal carcinoma have been reported in the literature [31]. These highly aggressive tumors represented 4% of all undifferentiated and poorly differentiated sinonasal carcinomas and 9% of tumors that have been previously classified as SNUC. This figure relates to archival diagnoses and not to the current classification system [31]. The majority of SMARCA4-deficient carcinomas develop in the nasal cavity, and they involve multiple sinonasal sites in a subset of cases. Because of their large monotonous cell morphology and their frequent neuroendocrine features, they have been frequently initially misclassified as neuroendocrine carcinomas. Histologically, SMARCA4-deficient carcinomas are undifferentiated, hence closely akin to SNUC. They are arranged in sheets of large anaplastic epithelioid cells disposed into irregularly communicating nests and lobules or trabeculae within a sparse to prominent reactive edematous or desmoplastic intervening stroma.
SMARCA4-deficient teratocarcinosarcomas
This rare multi-phenotypic (trilineage) and highly aggressive site-specific sinonasal malignancy is still defined by morphology in the current World Health Organization classification, but it merits mentioning here due to overlapping molecular features with SWI/SNF-deficient sinonasal carcinomas. Teratocarcinosarcoma (TCS) is defined by a triphasic growth of teratoma-like (embryonal epithelium of diverse types, neuroectodermal differentiation, and primitive neuroepithelium), carcinoma-like (either malignant differentiated epithelial elements or keratin-positive poorly differentiated proliferations), and sarcomatous stromal/mesenchymal elements with (mainly rhabdomyoblastic and rare osteochondroblastic) or without heterologous mesenchymal elements [34]. Recurrent loss of SMARCA4 expression in more than 80% of TCS cases was recently described [34]. However, in contrast to SWI/SNF-deficient sinonasal carcinoma types described above, where the SWI/SNF defect is definitional, sinonasal TCS is still defined morphologically based on the histological criteria set stated above [34].
SWI/SNF complex deficient sinonasal tumors are usually poorly differentiated or undifferentiated malignancies with a highly aggressive clinical course and poor patient outcomes. The mortality of SMARCA4-deficient sinonasal carcinomas is higher than in other tumors of this family [30, 35].
Differential diagnosis is challenging, and it is mainly defined by the histological pattern in any individual case. Non-keratinizing SCC (sporadic or HPV-related) and basaloid variants of many other entities such as NUT carcinoma, adamantinoma-like Ewing sarcoma, and neuroendocrine carcinomas must be considered. Large cell SWI/SNF-complex-deficient tumors need to be distinguished from SNUC, rare variants of NUT carcinoma, melanoma, dedifferentiated chordomas, aggressive anaplastic lymphomas, and metastases. The availability of immunohistochemical antibodies to SWI/SNF proteins represents an effective tool for the identification of these neoplasms in the appropriate clinicopathological and morphological context. SWI/SNF-complex-deficient malignancies can be determined by immunohistochemical staining with INI1 antibody for SMARCB1-deficient and BRG1 antibody for SMARCA4-deficient carcinomas. These antibodies are sensitive diagnostic tools (Fig. 3D) [31].
Regarding therapy, available data on the significance of the distinction between SMARCB1-deficient sinonasal carcinoma and adenocarcinoma as well as their long-term follow-up is limited. Currently, this distinction should enable reliable assessment of any therapeutic or prognostic differences between the two. Patients are often treated by radical surgery and adjuvant radiotherapy or radiochemotherapy. Despite this aggressive treatment, a recent multicenter case series revealed that only one of three patients was alive and free of tumors at a median follow-up of slightly more than 2 years. [25] The alternative therapeutic option rests on local control of the disease with polychemotherapy and radiotherapy [30, 35]. The largest single-institution study of SMARCB1-deficient sinonasal carcinoma to date reported the outcomes of 19 consecutive patients with SMARCB1 (INI-1)-deficient sinonasal carcinoma treated at the MD Anderson Cancer Center. The median overall survival (OS) and disease-free survival (DFS) were 31.8 and 9.9 months, respectively. Patients with nasal cavity or maxillary sinus tumors had 84% better disease-specific survival (DSS) (hazard ratio [HR], 0.136; 95% confidence interval [CI], 0.028–0.66; p = 0.005) and 71% better DFS (HR, 0.29; 95% CI, 0.097–0.84; p = 0.041) than patients with other sinonasal sites. Patients who received induction chemotherapy were 76% less likely to die of disease (DSS HR, 0.241; 95% CI, 0.058–1.00; p = 0.047) [36].
Recent findings suggest a promising role for immunomodulators and immune checkpoint inhibitors as potential drugs for patients with SWI/SNF-related malignancies [27, 37]. EZH2 inhibitor, a histone methyltransferase, activates the methylation of histone H3 at lysin 27 (H3K27me3), resulting in the epigenetic silencing of cell fate-associated genes. Tumor cells with loss of SMARCB1 demonstrate a constitutive EZH2 activation and oncogenic activation [27, 37]. EZH2 inhibitors may modulate tumor immunogenicity and anti-tumor immune responses [38].
Sinonasal undifferentiated carcinoma
Sinonasal undifferentiated carcinoma (SNUC) is a high-grade epithelial neoplasm without any lines of differentiation, and the diagnosis is made only after the exclusion of other sinonasal and non-sinonasal malignancies. In the previous WHO classifications, many undifferentiated epithelial neoplasms were included under this term, until advances in molecular pathology allowed for their identification as separate entities. Recently, isocitrate dehydrogenase 1 or 2 (IDH1/2) mutations were identified in a subset of SNUC [39, 40], including three main hotspot mutations of IDH1 R132, IDH2 R140, and IDH2 R172 [41]. Monoclonal or multi-specific antibodies for the detection of IDH1/2 mutations represent an alternative cheaper than molecular genetic testing, while immunohistochemistry using the present antibodies lacks the ability to detect the full spectrum of IDH1/2 mutations [42]. We recommend using both immunohistochemistry and molecular analysis. The IDH proteins participate in the Krebs cycle converting isocitrate to α-ketoglutarate. IDH mutations produce an oncometabolite, D-2-hydroxyglutarate (2-HG), that induces DNA hypermethylation.
SNUCs are aggressive neoplasms with a silent clinical course until they are diagnosed at an advanced stage, followed by a rapid progression and poor outcome. The standard therapeutical approach used to be a combination of surgery, chemotherapy, and radiation [43], but the optimal sequence of these treatments has been long debated. Recently, a landmark study on 95 previously untreated patients reported improved outcomes using a curative intent strategy of induction chemotherapy (IC). In SNUC patients who received this treatment, the 5-year DSS was 59% (95% CI, 53 to 66%). The response to IC determined whether concurrent chemoradiation was continued. Responders to IC with complete response (CR) or partial response (PR) showed an 81% 5-year DSS. Non-responders received surgery and postoperative radiotherapy and showed a 54% 5-year DSS [43, 44].
However, in patients who did not experience even a partial response to IC with concurrent chemoradiotherapy (CRT), the 5-year DSS was 0%. In patients without even partial response to IC and those who were treated with surgery plus radiotherapy or CRT, the 5-year DSS was 39% (adjusted hazard ratio of 5.68 [95% CI, 2.89 to 9.36]). The authors concluded that in patients who achieve a favorable response to IC, definitive CRT results in improved survival compared with those who undergo definitive surgery. In patients who do not achieve a favorable response to IC, surgery when feasible seems to provide a better chance of disease control and improved survival [44].
Tumors with IDH2 mutations show a better outcome than other SNUCs [43]. The presence of IDH mutations provides alternative therapeutic options including selective small molecule inhibitors (e.g. Enasidenib for IDH2 mutations and Ivosidenib for IDH1 mutations) which inhibit DNA hypermethylation and lead to delayed cancer cell growth and induction of cell differentiation [45].
HPV-associated multiphenotypic sinonasal carcinoma
HPV-associated multiphenotypic sinonasal carcinoma (HMSC) is an epithelial malignancy localized almost exclusively in the sinonasal tract and harboring high-risk HPV [46]. The most common serovariety is type 33, followed by other also rare serotypes such as 52, 56, and others. HMSC immunohistochemistry is positive for p16 and high-risk HPV testing using direct assays such as RNA in situ hybridization is also positive. A negative p16 result helps to exclude this tumor type, but positive p16 can be non-specific and cannot be used as an HPV surrogate in this tumor. HMSC is histologically very pleomorphic and may mimic different salivary and non-salivary tumor types. HMSCs are characterized by a dual population of ductal and myoepithelial cells, mainly solid growth patterns, areas of necrosis, as well as overlying involvement of the surface epithelium similar to high-grade dysplasia. In places, HMSC may histologically resemble adenoid cystic carcinoma (AdCC), which is an important differential diagnosis, as the prognosis of HMSC is usually good unlike true AdCC [47]. The histological appearance of HMSC is usually associated with high-grade cytomorphology and a destructive growth and propensity for local recurrence. Despite the aggressive appearance, HMSC has low metastatic potential and little tendency to lethal behavior [48].
Sinonasal adenoid cystic carcinoma
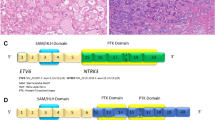
Adenoid cystic carcinoma (AdCC) is an invasive malignancy composed of epithelial and myoepithelial neoplastic cells arranged in tubular, cribriform, and solid patterns associated with an eosinophilic extracellular matrix and reduplicated basement membrane materials, and often with gene fusions involving the MYB, MYBL1, and NFIB genes. The genomic hallmarks of AdCC are t(6;9) or t(8;9) translocations, resulting in MYB::NFIB and MYBL1::NFIB fusions, respectively [49, 50] (Fig. 4A and B). The former alteration is found in > 80% of the cases and the latter in approximately 5% [50]. MYB/MYBL1 activation due to gene fusion or other mechanisms is a key event in the pathogenesis of AdCC [49]. Losses of 1p, 6q, and 15q are associated with high-grade tumors, while loss of 14q is seen exclusively in low-grade tumors [51, 52].
Sinonasal adenoid cystic carcinoma (AdCC). The fusions joining of MYB gene exon 14 with NFIB gene exon 9 (A) and MYBL1 gene exon 14 with NFIB gene exon 9 (B) are illustrated. Protein domains are depicted. Metatypical AdCC with prevailing solid pattern and focal squamous differentiation (C). Another unusual histological pattern of metatypical AdCC is a striking tubular hypereosinophilia (D)
Metatypical AdCC has been recently recognized as a morphological variant of AdCC with a predilection to the sinonasal mucosa [53, 54]. Metatypical AdCCs have been noted for unusual patterns, including squamous differentiation and macrocystic growth (Fig. 4C) [53]. Another unusual histological pattern of metatypical AdCC is a striking tubular hypereosinophilia and luminal cell prominence which are in contrast to the vast majority of AdCC that are basaloid with myoepithelial cell predominance (Fig. 4D) [54].
Next-generation sequencing of AdCC has identified mutations with mostly low levels of recurrence in genes of the FGF/IGF/PI3K, chromatin remodeling, and NOTCH signaling pathways [55, 56]. Traditional therapeutic agents for patients with advanced, recurrent, or metastatic AdCC have demonstrated poor efficacy in prolonging survival. Therefore, significant efforts have been undertaken to develop targeted therapies that could improve the outcome. In particular, the presence of NOTCH alterations in AdCC was shown to be associated with poor survival. Patients with these aberrations might potentially benefit from anti-NOTCH drugs, such as Brontictuzumab [57]. In the same line, AL101 (osugacestat) has been shown to have potent antitumor effects in in vitro and in vivo models (AdCC cell lines, organoids, and patient-derived xenograft models) of AdCC with activating NOTCH1 mutations [58].
In a very recent study, high expression of B7-H4 (VTCN1), a member of the B7 family, was associated with a poorer prognosis in AdCC, regardless of clinical stage and histologic subtype. B7-H4 expression was particularly high in solid ACC and was an independent prognostic marker in this disease [59]. Another recent study from MD Anderson Cancer Center demonstrated that the cooccurrence of multiple actionable protein/pathway alterations in various subtypes of AdCC indicates unique therapeutic vulnerabilities and opportunities for optimal combination therapy for this understudied and heterogeneous disease [60].
Soft tissue neoplasms with sinonasal predilection
Molecular alterations with differential diagnostic significance
The head and neck region can be a host to a wide range of soft tissue neoplasms, but a discussion of all these entities is beyond the scope of this review. Thus, we have included only soft tissue malignancies that occur frequently or are unique to the sinonasal tract with diagnostically useful molecular alterations. The discussed entities are listed in Table 2.
Biphenotypic sinonasal sarcoma
Biphenotypic sinonasal sarcoma (BSS) is a low-grade spindle cell mesenchymal neoplasm with neurogenic and myogenic differentiation localized exclusively in the sinonasal region. BSS is molecularly defined by the rearrangement of the PAX3 gene, which is involved in neurogenic, melanocytic, and skeletal muscle differentiation (Fig. 5 A–D) [61]. The most common gene fusion is PAX3::MAML3 in more than half of the cases, with alternative fusion partners including NCOA1, NCOA2, FOXO1, FOXO6, and WWTR1 [62,63,64].
Biphenotypic sinonasal sarcoma. Spindle cell proliferation with fascicular architecture of variable density, some areas are highly cellular (A). Hypocelular area with staghorn-like vessels resembling solitary fibrous tumor (B). The biphenotypic pattern is highlighted by S100 protein expression (C) and smooth muscle actin positivity (D)
BSS is usually a low-grade tumor with local recurrence reported in a third up to a half of the cases, and it may recur many years after diagnosis [65]. Three cases of BSS with high-grade transformation have been published [66,67,68], one of which developed into rhabdomyosarcoma (RMS). Molecular testing of BSS and RMS showed similar gene fusions including PAX3::FOXO1 and PAX3::NCOA1. Potentially, BSS and RMS form a continuum of lesions [62].
Ectomesenchymal chondromyxoid tumor
Ectomesenchymal chondromyxoid tumor (ECT) (aka RREB1::MRTFB-rearranged neoplasm) is a tumor of uncertain malignant potential located predominantly in the tongue and arising exceedingly rarely in extraglossal locations [69, 70]. The immunoprofile is non-specific and therefore molecular genetic testing is the preferable method when diagnosing ECT. This tumor is characterized in most cases by a RREB1::MRTFB fusion, while there is EWSR1 gene rearrangement in a smaller subset of cases [71, 72]. ECTs are genetically and histologically linked to soft tissue myoepithelial tumors [71] and may be mistaken for other mesenchymal tumors with dominant spindle cell morphology [73]. ECT usually follows a benign course with no metastasis. Surgery is curative in most cases, even though local recurrences may occur [69, 70]. Recently, neoplasms carrying the RREB1::MRTFB fusion and showing a variable morphological and immunophenotypic overlap with glossal ECT have been reported from diverse head and neck as well as from non-head and neck sites. Notably, 5 of 10 reported cases, reviewed in [73], involved the parapharyngeal space and the mandible/or sinonasal tract. One reported sinonasal tumor carrying the RREB1::MRTFB fusion was more similar to sinonasal chondromesenchymal hamartoma but lacked DICER1 alterations known to characterize the majority of sinonasal chondromesenchymal hamartomas [73].
GLI1-altered soft tissue tumors
GLI1-altered soft tissue neoplasms are recently described mesenchymal tumors of uncertain histogenesis, characterized by epithelioid or glomoid (and less frequently focally spindled) morphology and non-specific immunoprofile, presenting in the head and neck in 40% of cases. In two-thirds of the cases, these tumors harbor GLI1 fusions including ACTB::GLI1, PTCH1::GLI1, MALAT1::GLI1, and DERA::GLI1, while the rest harbor GLI1 amplification [1, 74, 75]. While the various fusion partners to GLI1 are best determined by targeted RNA-sequencing, the GLI1 amplification can also be detected by FISH. The possibility of co-amplification of the neighboring genes (particularly CDK4, MDM2, STAT6, and DDIT3) on chromosome 12 and detected by FISH has to be taken into account [76]. In addition, GLI1 immunostaining shows high specificity and good sensitivity for GLI1-rearranged mesenchymal tumors [77]. The combination of GLI1 IHC and p16 stain is superior in detecting GLI1 amplified neoplasms [78].
The biologic behavior of GLI1-altered soft tissue neoplasms varies from completely indolent to potentially aggressive metastasizing neoplasms. Local recurrence or distant spread may appear in approximately 20% of the cases [75, 79]. Potential targeted therapeutic options in GLI1-altered neoplasms include sonic hedgehog signaling pathway inhibitors [80].
Sinonasal rhabdomyosarcomas
Rhabdomyosarcomas (RMS) are clinically, prognostically, and biologically heterogeneous groups of tumors categorized morphologically into four main subtypes [81]. However, molecular-genetic findings delineate six distinct subtypes; embryonal RMS with unknown driver mutations/fusions, alveolar RMS with FOXO1 fusions, MYOD1-mutated RMS with MYOD1 activating mutations, VGLL2/VGLL3/NCOA2-rearranged RMS, pleomorphic RMS with a complex genetic background, and finally, TFCP2-rearranged RMS with EWSR1 or FUS fusion partners [82]. Spindle cell/sclerosing RMS are usually positive (at least focally) for cytokeratins and the myogenic markers, mainly MyoD1, myogenin, desmin, or PAX7. The combination of positivity for pankeratin, ALK, and desmin is highly suggestive of TFCP2-fused RMS.
VGLL2/VGLL3/NCOA2-rearranged RMS belong to a category of spindle cell/sclerosing RMS, primarily affecting newborns or infants and exhibiting head and neck predilection. The characteristic molecular genetic events involve VGLL2::CITED3, VGLL2::NCOA2, TEAD1::NCOA2, or SRF::NCOA2 gene fusions [83, 84]. Recently, six cases with novel VGLL3 rearrangements with TCF12, EP300, and PPARGC1A as fusion partners have been described [85]. Clinically, these tumors have a favorable prognosis. Only four cases with metastases have been reported. Upon relapse, three of them displayed high-grade morphology with mutations in genes encoding cell cycle proteins (particularly TP53, CDKN2A/B, and FGFR4) [86]. Complete surgical resection is usually curative, while RMS-type chemotherapy is recommended for unresectable cases because of the potential for high-grade transformation.
TFCP2-rearranged RMS (TFCP2-RMS) is a spindle cell/sclerosing and very aggressive mesenchymal tumor with rhabdomyoblastic differentiation, characterized by EWSR1/FUS::TFCP2 rearrangements [87]. In a subset of cases, a hemizygous deletion or amplification of the ALK gene has been described [88]. TFCP2-RMS tumors are predominantly localized in the jaws and the skull of young adults, often with secondary involvement of soft tissues. The tumors are usually diagnosed at an advanced stage and have a rapid clinical course with a dismal prognosis and a 3-year overall survival of 28% despite aggressive multimodal therapy [89]. Treatment options include surgery which may not be indicated if massive tumor spread has occurred by the time of diagnosis. Chemotherapy and radiotherapy do not prolong survival. The combination of radiation and ALK inhibitors (crizotinib, alectinib, lorlatinib, and/or pazopanib) has shown partial response in anecdotal cases [90, 91].
Adamantinoma-like Ewing sarcoma
Adamantinoma-like Ewing sarcoma (ALES) is a controversial variant of Ewing sarcoma (ES) defined by the presence of t(11;22) and EWSR1::FLI1 fusion [92]. It is speculated whether it represents an epithelial or mesenchymal neoplasm, as epithelial markers (pan-cytokeratins and p63/p40) and ES-related markers (CD99 and NKX2.2) are commonly expressed [93]. It is uncertain whether this tumor should be managed according to carcinoma or sarcoma protocol. Many tumors are treated with surgery and adjuvant polychemotherapy according to ES-specific protocols.
EWSR1/FUS::POU2AF3(COLCA2) sarcomas
First reported by Agaimy et al. [94], EWSR1/FUS::POU2AF3(COLCA2) sarcomas are newly recognized aggressive neoplasms that exhibit a propensity to both local recurrence and metastatic spread despite multimodal treatment. They affect adult patients and commonly arise in the head and neck, with a striking predilection for the sinonasal tract. Their morphological spectrum ranges from spindle cell proliferation with mild nuclear atypia, through biphasic tumors composed of sheets and fascicles of spindled and round cells with features of neuroendocrine differentiation, to purely round cell tumors with high nuclear grade (Fig. 6A–D) [94,95,96]. In addition, some tumors were reported to contain foci of glandular, rhabdomyoblastic, or osteogenic differentiation [95]. Approximately half of the cases display pan-cytokeratin positivity [94,95,96], possibly leading to diagnostic confusion with synovial sarcoma. In general, EWSR1 fusions with various partners are frequent in soft tissue tumors and include rare instances of alternative EWSR1::SSX1 fusion in synovial sarcoma [97], or potential FUS fusion with POU2AF3 instead of EWSR1 [96]. Consequently, FISH detection of an EWSR1 break is not a sufficient diagnostic test. Instead, targeted RNA sequencing is advised to render the correct diagnosis.
EWSR1/FUS::POU2AF3(COLCA2) sarcomas. The tumor consists of solid tumor nests with a neuroendocrine tumor-like appearance composed of basaloid tumor cells with scattered stroma (A). Extensive perineural spread is common (B). Weak OSCAR expression is occasionally present, the intensity of which can be compared with the strong expression of the mucosal epithelium (lower-right corner) (C). CD56 is an unspecific marker but is frequently expressed (D)
Summary
In conclusion, with the widespread application of molecular testing, there has been a rapid development in the classification of head and neck tumors in general, but clearly also in the field of tumors of the sinonasal area and the anterior skull base. The availability of an expanding spectrum of immunohistochemical surrogates provides the pathologist with tools for rapid identification of diagnostic molecular abnormalities, that, for the clinicians, increasingly allow the selection of adequate targeted therapies. While still largely relying on histology and immunohistochemistry for diagnosis, the new insights into the molecular basis of sinonasal tumors clearly allow pathologists nowadays to increase their diagnostic accuracy, allowing for better fine-tuning and eliminating overlapping diagnoses. It is fair to say that the adoption of the molecular biological basis of sinonasal malignancies in the 5th edition of the WHO classification reflects the irreversible place these techniques have conquered in the daily practice of head and neck cancer departments.
Data availability
Data supporting the findings of this study are available within the article. The complete datasets generated during and/or analyzed during the current study are available from the corresponding author upon reasonable request.
Code availability
Not applicable.
References
WHO Classification of Tumours Editorial Board. Head and neck tumours. Lyon (France): International Agency for Research on Cancer; forthcoming. (WHO classification of tumours series, 5th edn, vol 9). https://publications.iarc.fr
Chatelet F, Simon F, Bedarida V, Le Clerc N, Adle-Biassette H, Manivet P, Herman P, Verillaud B (2021) Surgical management of sinonasal cancers: a comprehensive review. Cancers (Basel) 13(16):3995. https://doi.org/10.3390/cancers13163995
Bossi P, Orlandi E, Resteghini C et al (2023) The SINTART 2 study. A phase II non-randomised controlled trial of induction chemotherapy, photon-, proton- and carbon-ion-based radiotherapy integration in patients with locally advanced unresectable sinonasal tumours. Eur J Cancer 187:134–143
Yang W, Lee KW, Srivastava RM et al (2019) Immunogenic neoantigens derived from gene fusions stimulate T cell responses. Nat Med 25:767–775
Todorovic E, Truong T, Eskander A et al (2020) Middle ear and temporal bone nonkeratinizing squamous cell carcinomas with DEK-AFF2 fusion: an emerging entity. Am J Surg Pathol 44:1244–1250
Ruangritchankul K, Sandison A (2023) DEK::AFF2 fusion carcinomas of head and neck. Adv Anat Pathol 30:86–94
Rooper LM, Agaimy A, Dickson BC et al (2021) DEK-AFF2 carcinoma of the sinonasal region and skull base: detailed clinicopathologic characterization of a distinctive entity. Am J Surg Pathol 45:1682–1693
Kuo YJ, Lewis JS Jr, Zhai C et al (2021) DEK-AFF2 fusion-associated papillary squamous cell carcinoma of the sinonasal tract: clinicopathologic characterization of seven cases with deceptively bland morphology. Mod Pathol 34:1820–1830
Bishop JA, Gagan J, Paterson C et al (2021) Nonkeratinizing squamous cell carcinoma of the sinonasal tract with DEK-AFF2: further solidifying an emerging entity. Am J Surg Pathol 45:718–720
Kuo YJ, Lewis JS Jr, Truong T et al (2022) Nuclear expression of AFF2 C-terminus is a sensitive and specific ancillary marker for DEK::AFF2 carcinoma of the sinonasal tract. Mod Pathol 35:1587–1595
Hellquist H, French CA, Bishop JA et al (2017) NUT midline carcinoma of the larynx: an international series and review of the literature. Histopathology 70:861–868
Chau NG, Ma C, Danga K et al (2020) An anatomical site and genetic-based prognostic model for patients with nuclear protein in testis (NUT) midline carcinoma: analysis of 124 patients. JNCI Cancer Spectr 4:pkz094
Haack H, Johnson LA, Fry CJ et al (2009) Diagnosis of NUT midline carcinoma using a NUT-specific monoclonal antibody. Am J Surg Pathol 33:984–991
Le Loarer F, Pissaloux D, Watson S et al (2019) Clinicopathologic features of CIC-NUTM1 sarcomas, a new molecular variant of the family of CIC-fused sarcomas. Am J Surg Pathol 43:268–276
Schaefer IM, Dal Cin P, Landry LM et al (2018) CIC-NUTM1 fusion: a case which expands the spectrum of NUT-rearranged epithelioid malignancies. Genes Chromosom Cancer 57:446–451
French CA, Miyoshi I, Kubonishi I et al (2003) BRD4-NUT fusion oncogene: a novel mechanism in aggressive carcinoma. Cancer Res 63:304–307
Pivot-Pajot C, Caron C, Govin J et al (2003) Acetylation-dependent chromatin reorganization by BRDT, a testis-specific bromodomain-containing protein. Mol Cell Biol 23:5354–5365
French CA, Ramirez CL, Kolmakova J et al (2008) BRD-NUT oncoproteins: a family of closely related nuclear proteins that block epithelial differentiation and maintain the growth of carcinoma cells. Oncogene 27:2237–2242
Huang QW, He LJ, Zheng S et al (2019) An overview of molecular mechanism, clinicopathological factors, and treatment in NUT carcinoma. Biomed Res Int 2019:1018439
French CA, Rahman S, Walsh EM et al (2014) NSD3-NUT fusion oncoprotein in NUT midline carcinoma: implications for a novel oncogenic mechanism. Cancer Discov 4:928–941
Alekseyenko AA, Walsh EM, Zee BM et al (2017) Ectopic protein interactions within BRD4-chromatin complexes drive oncogenic megadomain formation in NUT midline carcinoma. Proc Natl Acad Sci U S A 114:E4184–E4192
Bauer DE, Mitchell CM, Strait KM et al (2012) Clinicopathologic features and long-term outcomes of NUT midline carcinoma. Clin Cancer Res 18:5773–5779
Cheng ML, Huang Y, Luong N et al (2023) Exceptional response to bromodomain and extraterminal domain inhibitor therapy with BMS-986158 in BRD4-NUTM1 NUT carcinoma harboring a BRD4 splice site mutation. JCO Precis Oncol 7:e2200633
Ramesh U, Contrera KJ, Shakibai N et al (2024) Sinonasal NUT carcinoma: a consecutive case series and systematic review. Head Neck 46:29–36
Filippakopoulos P, Qi J, Picaud S et al (2010) Selective inhibition of BET bromodomains. Nature 468:1067–1073
Tontsch-Grunt U, Traexler PE, Baum A et al (2022) Therapeutic impact of BET inhibitor BI 894999 treatment: backtranslation from the clinic. Br J Cancer 127:577–586
Wang X, Haswell JR, Roberts CW (2014) Molecular pathways: SWI/SNF (BAF) complexes are frequently mutated in cancer–mechanisms and potential therapeutic insights. Clin Cancer Res 20:21–27
Agaimy A, Koch M, Lell M et al (2014) SMARCB1(INI1)-deficient sinonasal basaloid carcinoma: a novel member of the expanding family of SMARCB1-deficient neoplasms. Am J Surg Pathol 38:1274–1281
Bishop JA, Antonescu CR, Westra WH (2014) SMARCB1 (INI-1)-deficient carcinomas of the sinonasal tract. Am J Surg Pathol 38:1282–1289
Agaimy A, Hartmann A, Antonescu CR et al (2017) SMARCB1 (INI-1)-deficient sinonasal carcinoma: a series of 39 cases expanding the morphologic and clinicopathologic spectrum of a recently described entity. Am J Surg Pathol 41:458–471
Agaimy A (2023) SWI/SNF-deficient sinonasal carcinomas. Adv Anat Pathol 30:95–103
Skálová A, Taheri T, Bradová M, Vaněček T, Franchi A, Slouka D, Kostlivý T, de Rezende G, Michálek J, Klubíčková N, Ptáková N, Nemcová A, Michal M, Agaimy A, Leivo I (2023) SMARCB1-deficient sinonasal adenocarcinoma: a rare variant of SWI/SNF-deficient malignancy often misclassified as high-grade non-intestinal-type sinonasal adenocarcinoma or myoepithelial carcinoma. Virchows Arch. https://doi.org/10.1007/s00428-023-03650-2
Agaimy A, Weichert W (2017) SMARCA4-deficient sinonasal carcinoma. Head Neck Pathol 11:541–545
Rooper LM, Uddin N, Gagan J et al (2020) Recurrent loss of SMARCA4 in sinonasal teratocarcinosarcoma. Am J Surg Pathol 44:1331–1339
Agaimy A, Jain D, Uddin N et al (2020) SMARCA4-deficient sinonasal carcinoma: a series of 10 cases expanding the genetic spectrum of SWI/SNF-driven sinonasal malignancies. Am J Surg Pathol 44:703–710
Contrera KJ, Shakibai N, Su SY et al (2023) Impact of clinical factors and treatments on SMARCB1 (INI-1)-Deficient Sinonasal Carcinoma. Otolaryngol Head Neck Surg 169:435–440
Ngo C, Postel-Vinay S (2022) Immunotherapy for SMARCB1-deficient sarcomas: current evidence and future developments. Biomedicines 10(3):650. https://doi.org/10.3390/biomedicines10030650
Aspeslagh S, Morel D, Soria JC et al (2018) Epigenetic modifiers as new immunomodulatory therapies in solid tumours. Ann Oncol 29:812–824
Jo VY, Chau NG, Hornick JL et al (2017) Recurrent IDH2 R172X mutations in sinonasal undifferentiated carcinoma. Mod Pathol 30:650–659
Dogan S, Chute DJ, Xu B et al (2017) Frequent IDH2 R172 mutations in undifferentiated and poorly-differentiated sinonasal carcinomas. J Pathol 242:400–408
Riobello C, Lopez-Hernandez A, Cabal VN et al (2020) IDH2 Mutation analysis in undifferentiated and poorly differentiated sinonasal carcinomas for diagnosis and clinical management. Am J Surg Pathol 44:396–405
Mito JK, Bishop JA, Sadow PM et al (2018) Immunohistochemical detection and molecular characterization of IDH-mutant sinonasal undifferentiated carcinomas. Am J Surg Pathol 42:1067–1075
Chambers KJ, Lehmann AE, Remenschneider A et al (2015) Incidence and survival patterns of sinonasal undifferentiated carcinoma in the United States. J Neurol Surg B Skull Base 76:94–100
Amit M, Abdelmeguid AS, Watcherporn T et al (2019) Induction chemotherapy response as a guide for treatment optimization in sinonasal undifferentiated carcinoma. J Clin Oncol 37:504–512
Stein EM, DiNardo CD, Fathi AT et al (2021) Ivosidenib or enasidenib combined with intensive chemotherapy in patients with newly diagnosed AML: a phase 1 study. Blood 137:1792–1803
Bishop JA, Ogawa T, Stelow EB et al (2013) Human papillomavirus-related carcinoma with adenoid cystic-like features: a peculiar variant of head and neck cancer restricted to the sinonasal tract. Am J Surg Pathol 37:836–844
Bishop JA, Andreasen S, Hang JF et al (2017) HPV-related multiphenotypic sinonasal carcinoma: an expanded series of 49 cases of the tumor formerly known as HPV-related carcinoma with adenoid cystic carcinoma-like features. Am J Surg Pathol 41:1690–1701
Rodarte AI, Parikh AS, Gadkaree SK et al (2019) Human papillomavirus related multiphenotypic sinonasal carcinoma: report of a case with early and progressive metastatic disease. J Neurol Surg Rep 80:e41–e43
Persson M, Andren Y, Mark J et al (2009) Recurrent fusion of MYB and NFIB transcription factor genes in carcinomas of the breast and head and neck. Proc Natl Acad Sci U S A 106:18740–18744
Fujii K, Murase T, Beppu S et al (2017) MYB, MYBL1, MYBL2 and NFIB gene alterations and MYC overexpression in salivary gland adenoid cystic carcinoma. Histopathology 71:823–834
Persson M, Andren Y, Moskaluk CA et al (2012) Clinically significant copy number alterations and complex rearrangements of MYB and NFIB in head and neck adenoid cystic carcinoma. Genes Chromosom Cancer 51:805–817
Steiner P, Andreasen S, Grossmann P et al (2018) Prognostic significance of 1p36 locus deletion in adenoid cystic carcinoma of the salivary glands. Virchows Arch 473:471–480
Mathew EP, Todorovic E, Truong T et al (2022) Metatypical adenoid cystic carcinoma: a variant showing prominent squamous differentiation with a predilection for the sinonasal tract and skull base. Am J Surg Pathol 46:816–822
Weinreb I, Rooper LM, Dickson BC et al (2023) Adenoid cystic carcinoma with striking tubular hypereosinophilia: a unique pattern associated with nonparotid location and both canonical and novel EWSR1::MYB and FUS::MYB fusions. Am J Surg Pathol 47:497–503
Ho AS, Kannan K, Roy DM et al (2013) The mutational landscape of adenoid cystic carcinoma. Nat Genet 45:791–798
Ho AS, Ochoa A, Jayakumaran G et al (2019) Genetic hallmarks of recurrent/metastatic adenoid cystic carcinoma. J Clin Invest 129:4276–4289
Miller LE, Au V, Mokhtari TE, Goss D, Faden DL, Varvares MA (2022) A contemporary review of molecular therapeutic targets for adenoid cystic carcinoma. Cancers (Basel) 14(4):992. https://doi.org/10.3390/cancers14040992
Ferrarotto R, Mishra V, Herz E et al (2022) AL101, a gamma-secretase inhibitor, has potent antitumor activity against adenoid cystic carcinoma with activated NOTCH signaling. Cell Death Dis 13:678
Siqueira JM, Mitani Y, Hoff CO et al (2023) Analysis of B7–H4 expression across salivary gland carcinomas reveals adenoid cystic carcinoma-specific prognostic relevance. Mod Pathol 37:100371
Ferrarotto R, Mitani Y, McGrail DJ et al (2021) Proteogenomic analysis of salivary adenoid cystic carcinomas defines molecular subtypes and identifies therapeutic targets. Clin Cancer Res 27:852–864
Wang X, Bledsoe KL, Graham RP et al (2014) Recurrent PAX3-MAML3 fusion in biphenotypic sinonasal sarcoma. Nat Genet 46:666–668
Fritchie KJ, Jin L, Wang X et al (2016) Fusion gene profile of biphenotypic sinonasal sarcoma: an analysis of 44 cases. Histopathology 69:930–936
Le Loarer F, Laffont S, Lesluyes T et al (2019) Clinicopathologic and molecular features of a series of 41 biphenotypic sinonasal sarcomas expanding their molecular spectrum. Am J Surg Pathol 43:747–754
Nichols MM, Alruwaii F, Chaaban M et al (2023) Biphenotypic sinonasal sarcoma with a novel PAX3::FOXO6 fusion: a case report and review of the literature. Head Neck Pathol 17:259–264
Kominsky E, Boyke AE, Madani D et al (2023) Biphenotypic sinonasal sarcoma: a case report and review of literature. Ear Nose Throat J 102:385–390
Bell D, Phan J, DeMonte F et al (2022) High-grade transformation of low-grade biphenotypic sinonasal sarcoma: radiological, morphophenotypic variation and confirmatory molecular analysis. Ann Diagn Pathol 57:151889
Hasnie S, Glenn C, Peterson JEG et al (2022) High-grade biphenotypic sinonasal sarcoma: a case report. J Neurol Surg Rep 83:e105–e109
Meyer A, Klubickova N, Mosaieby E et al (2023) Biphenotypic sinonasal sarcoma with PAX3::MAML3 fusion transforming into high-grade rhabdomyosarcoma: report of an emerging rare phenomenon. Virchows Arch 482:777–782
Smith BC, Ellis GL, Meis-Kindblom JM et al (1995) Ectomesenchymal chondromyxoid tumor of the anterior tongue. Nineteen cases of a new clinicopathologic entity. Am J Surg Pathol 19:519–530
Bubola J, Hagen K, Blanas N et al (2021) Expanding awareness of the distribution and biologic potential of ectomesenchymal chondromyxoid tumor. Head Neck Pathol 15:319–322
Argyris PP, Bilodeau EA, Yancoskie AE et al (2016) A subset of ectomesenchymal chondromyxoid tumours of the tongue show EWSR1 rearrangements and are genetically linked to soft tissue myoepithelial neoplasms: a study of 11 cases. Histopathology 69:607–613
Dickson BC, Antonescu CR, Argyris PP et al (2018) Ectomesenchymal chondromyxoid tumor: a neoplasm characterized by recurrent RREB1-MKL2 fusions. Am J Surg Pathol 42:1297–1305
Agaimy A, Din NU, Dermawan JK et al (2023) RREB1::MRTFB fusion-positive extra-glossal mesenchymal neoplasms: a series of five cases expanding their anatomic distribution and highlighting significant morphological and phenotypic diversity. Genes Chromosom Cancer 62:5–16
Dahlen A, Fletcher CD, Mertens F et al (2004) Activation of the GLI oncogene through fusion with the beta-actin gene (ACTB) in a group of distinctive pericytic neoplasms: pericytoma with t(7;12). Am J Pathol 164:1645–1653
Papke DJ Jr, Dickson BC, Oliveira AM et al (2023) Distinctive nested glomoid neoplasm: clinicopathologic analysis of 20 cases of a mesenchymal neoplasm with frequent GLI1 alterations and indolent behavior. Am J Surg Pathol 47:12–24
Agaram NP, Zhang L, Sung YS et al (2019) GLI1-amplifications expand the spectrum of soft tissue neoplasms defined by GLI1 gene fusions. Mod Pathol 32:1617–1626
Parrack PH, Marino-Enriquez A, Fletcher CDM et al (2023) GLI1 Immunohistochemistry distinguishes mesenchymal neoplasms with GLI1 alterations from morphologic mimics. Am J Surg Pathol 47:453–460
Machado I, Agaimy A, Giner F, Navarro S, Michal M, Bridge J, Claramunt R, López-Guerrero JA, Alcacer J, Linos K, Llombart-Bosch A (2023) The value of GLI1 and p16 immunohistochemistry in the premolecular screening for GLI1-altered mesenchymal neoplasms. Virchows Arch. https://doi.org/10.1007/s00428-023-03687-3
Xu B, Chang K, Folpe AL et al (2020) Head and neck mesenchymal neoplasms with GLI1 gene alterations: a pathologic entity with distinct histologic features and potential for distant metastasis. Am J Surg Pathol 44:729–737
Antonescu CR, Agaram NP, Sung YS et al (2018) A distinct malignant epithelioid neoplasm with GLI1 gene rearrangements, frequent S100 protein expression, and metastatic potential: expanding the spectrum of pathologic entities with ACTB/MALAT1/PTCH1-GLI1 fusions. Am J Surg Pathol 42:553–560
WHO Classification of Tumours Editorial Board. Soft tissue and bone tumours. Lyon (France): International Agency for Research on Cancer; 2020. (WHO classification of tumours series, 5th edn, vol 3). https://publications.iarc.fr/588
Leiner J, Le Loarer F (2020) The current landscape of rhabdomyosarcomas: an update. Virchows Arch 476:97–108
Mosquera JM, Sboner A, Zhang L et al (2013) Recurrent NCOA2 gene rearrangements in congenital/infantile spindle cell rhabdomyosarcoma. Genes Chromosom Cancer 52:538–550
Alaggio R, Zhang L, Sung YS et al (2016) A molecular study of pediatric spindle and sclerosing rhabdomyosarcoma: identification of novel and recurrent VGLL2-related fusions in infantile cases. Am J Surg Pathol 40:224–235
Agaimy A, Dermawan JK, Leong I et al (2022) Recurrent VGLL3 fusions define a distinctive subset of spindle cell rhabdomyosarcoma with an indolent clinical course and striking predilection for the head and neck. Genes Chromosom Cancer 61:701–709
Cyrta J, Gauthier A, Karanian M et al (2021) Infantile rhabdomyosarcomas with VGLL2 rearrangement are not always an indolent disease: a study of 4 aggressive cases with clinical, pathologic, molecular, and radiologic findings. Am J Surg Pathol 45:854–867
Watson S, Perrin V, Guillemot D et al (2018) Transcriptomic definition of molecular subgroups of small round cell sarcomas. J Pathol 245:29–40
Le Loarer F, Cleven AHG, Bouvier C et al (2020) A subset of epithelioid and spindle cell rhabdomyosarcomas is associated with TFCP2 fusions and common ALK upregulation. Mod Pathol 33:404–419
Dehner CA, Broski SM, Meis JM et al (2023) Fusion-driven spindle cell rhabdomyosarcomas of bone and soft tissue: a clinicopathologic and molecular genetic study of 25 cases. Mod Pathol 36:100271
Brunac AC, Laprie A, Castex MP et al (2020) The combination of radiotherapy and ALK inhibitors is effective in the treatment of intraosseous rhabdomyosarcoma with FUS-TFCP2 fusion transcript. Pediatr Blood Cancer 67:e28185
Valerio E, Furtado Costa JL, Perez Fraile NM et al (2023) Intraosseous spindle cell/epithelioid rhabdomyosarcoma with tfcp2 rearrangement: a recent recognized subtype with partial response to alectinib. Int J Surg Pathol 31:861–865
Bridge JA, Fidler ME, Neff JR et al (1999) Adamantinoma-like Ewing’s sarcoma: genomic confirmation, phenotypic drift. Am J Surg Pathol 23:159–165
Rooper LM, Bishop JA (2020) Soft tissue special issue: Adamantinoma-like Ewing sarcoma of the head and neck: a practical review of a challenging emerging entity. Head Neck Pathol 14:59–69
Agaimy A, Baneckova M, De Almeida J et al (2023) Recurrent EWSR1::COLCA2 fusions define a novel sarcoma with spindle/round cell morphology and strong predilection for the sinonasal tract. Am J Surg Pathol 47:361–369
Koshyk O, Dehner CA, van den Hout MFCM, Bempt IV, Sciot R, Huang HY, Agaimy A, Din NU, Klubíčková N, Mosaieby E, Skálová A, Michalová K, Schöffski P, Oliveira AM, Halling KC, Gupta S, Gross JM, Nin JWM, Michal M, Folpe AL, Kosemehmetoglu K, Torres-Mora J, Michal M (2023) EWSR1::POU2AF3(COLCA2) sarcoma: an aggressive, polyphenotypic sarcoma with a head and neck predilection. Mod Pathol 36(12):100337. https://doi.org/10.1016/j.modpat.2023.100337
Hiemenz MC, Kaur J, Kuang Z et al (2023) POU2AF3-rearranged sarcomas: a novel tumor defined by fusions of EWSR1 or FUS to a gene formerly designated COLCA2. Genes Chromosom Cancer 62:460–470
Yoshida A, Arai Y, Satomi K et al (2022) Identification of novel SSX1 fusions in synovial sarcoma. Mod Pathol 35:228–239
Funding
Open access publishing supported by the National Technical Library in Prague. This study was supported by a study grant from the Cooperation Program, research area SURG (Alena Skálová, Martina Bradová), the project National Institute for Cancer Research—NICR (Programme EXCELES, ID project no. LX22NPO5102)—funded by the European Union—Next Generation EU (Alena Skálová, Martina Bradová), the Turku University Hospital Fund, the Maritza and Reino Salonen Foundation, and the Finnish Cancer Society, Finland (Ilmo Leivo).
Author information
Authors and Affiliations
Contributions
All authors contributed to the study’s conception and design. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval
Not applicable.
Informed consent
No patient consent was required for this review article.
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Alfio Ferlito and Ilmo Leivo are senior authors of this manuscript with equal contributions.
“This article was written by members and invitees of the International Head and Neck Scientific Group (www.IHNSG.com)”.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Skálová, A., Agaimy, A., Bradova, M. et al. Molecularly defined sinonasal malignancies: an overview with focus on the current WHO classification and recently described provisional entities. Virchows Arch 484, 885–900 (2024). https://doi.org/10.1007/s00428-024-03775-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00428-024-03775-y