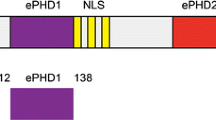
Abstract
Members of the tristetraprolin (TTP) family of RNA binding proteins (RBPs) regulate the metabolism of a variety of mRNA targets. In mammals, these proteins modulate many physiological processes, including immune cell activation, hematopoiesis, and embryonic development. Regulation of mRNA stability by these proteins requires that the tandem zinc finger (TZF) domain binds initially and directly to target mRNAs, ultimately leading to their deadenylation and decay. Proteins of this type throughout eukarya possess a highly conserved TZF domain, suggesting that they are all capable of high-affinity RNA binding. However, the mechanism of TTP-mediated mRNA decay is largely undefined. Given the vital role that these TTP family proteins play in maintaining RNA homeostasis throughout eukaryotes, we focused here on the first, key step in this process: recognition and binding of the TZF domain to target RNA. For these studies, we chose a primitive plant, the spikemoss Selaginella moellendorffii, which last shared a common ancestor with humans more than a billion years ago. Here we report the near complete backbone and side chain resonance assignments of the spikemoss TZF domain, including: (1) the assignment of the RNA-TZF domain complex, representing one of only two data sets currently available for the entire TTP family of proteins; and (2) the first NMR resonance assignments of the entire TZF domain, in the RNA-free form. This work will serve as the basis for further NMR structural investigations aimed at gaining insights into the process of RNA recognition and the mechanisms of TTP-mediated mRNA decay.


Similar content being viewed by others
References
Amann BT, Worthington MT, Berg JM (2003) A Cys3His zinc-binding domain from Nup475/tristetraprolin: a novel fold with a disklike structure. Biochemistry 42:217–221. https://doi.org/10.1021/bi026988m
Bai W, Wells ML, Lai WS, Hicks SN, Burkholder AB, Perera L, Kimmel AR, Blackshear PJ (2021) A post-transcriptional regulon controlled by TtpA, the single tristetraprolin family member expressed in Dictyostelium discoideum. Nucleic Acids Res 49:11920–11937. https://doi.org/10.1093/nar/gkab983
Blackshear PJ, Lai WS, Kennington EA, Brewer G, Wilson GM, Guan X, Zhou P (2003) Characteristics of the interaction of a synthetic human tristetraprolin tandem zinc finger peptide with AU-rich element-containing RNA substrates. J Biol Chem 278:19947–19955. https://doi.org/10.1074/jbc.M301290200
Carballo E, Lai WS, Blackshear PJ (2000) Evidence that tristetraprolin is a physiological regulator of granulocyte-macrophage colony-stimulating factor messenger RNA deadenylation and stability. Blood 95:1891–1899
Choi YJ, Lai WS, Fedic R, Stumpo DJ, Huang W, Li L, Perera L, Brewer BY, Wilson GM, Mason JM, Blackshear PJ (2014) The drosophila Tis11 protein and its effects on mRNA expression in flies. J Biol Chem 289:35042–35060. https://doi.org/10.1074/jbc.M114.593491
Coggins BE, Zhou P (2008) High resolution 4-D spectroscopy with sparse concentric shell sampling and FFT-CLEAN. J Biomol NMR 42:225–239. https://doi.org/10.1007/s10858-008-9275-x
Cuthbertson BJ, Liao Y, Birnbaumer L, Blackshear PJ (2008) Characterization of zfs1 as an mRNA-binding and -destabilizing protein in Schizosaccharomyces pombe. J Biol Chem 283:2586–2594. https://doi.org/10.1074/jbc.M707154200
Delaglio F, Grzesiek S, Vuister GW, Zhu G, Pfeifer J, Bax A (1995) NMRPipe: a multidimensional spectral processing system based on UNIX pipes. J Biomol NMR 6:277–293. https://doi.org/10.1007/bf00197809
Fabian MR, Frank F, Rouya C, Siddiqui N, Lai WS, Karetnikov A, Blackshear PJ, Nagar B, Sonenberg N (2013) Structural basis for the recruitment of the human CCR4-NOT deadenylase complex by tristetraprolin. Nat Struct Mol Biol 20:735–739. https://doi.org/10.1038/nsmb.2572
Hudson BP, Martinez-Yamout MA, Dyson HJ, Wright PE (2004) Recognition of the mRNA AU-rich element by the zinc finger domain of TIS11d. Nat Struct Mol Biol 11:257–264. https://doi.org/10.1038/nsmb738
Johnson BA, Blevins RA (1994) NMR view: a computer program for the visualization and analysis of NMR data. J Biomol NMR 4:603–614. https://doi.org/10.1007/bf00404272
Lai WS, Carballo E, Strum JR, Kennington EA, Phillips RS, Blackshear PJ (1999) Evidence that tristetraprolin binds to AU-rich elements and promotes the deadenylation and destabilization of tumor necrosis factor alpha mRNA. Mol Cell Biol 19:4311–4323. https://doi.org/10.1128/mcb.19.6.4311
Lai WS, Carballo E, Thorn JM, Kennington EA, Blackshear PJ (2000) Interactions of CCCH zinc finger proteins with mRNA. Binding of tristetraprolin-related zinc finger proteins to Au-rich elements and destabilization of mRNA. J Biol Chem 275:17827–17837. https://doi.org/10.1074/jbc.M001696200
Lai WS, Wells ML, Perera L, Blackshear PJ (2019a) The tandem zinc finger RNA binding domain of members of the tristetraprolin protein family. Wiley Interdiscip Rev RNA 10:e1531. https://doi.org/10.1002/wrna.1531
Lai WS, Stumpo DJ, Wells ML, Gruzdev A, Hicks SN, Nicholson CO, Yang Z, Faccio R, Webster MW, Passmore LA, Blackshear PJ (2019b) Importance of the conserved carboxyl-terminal CNOT1 binding domain to tristetraprolin activity in vivo. Mol Cell Biol. https://doi.org/10.1128/MCB.00029-19
Lee W, Markley JL (2018) PINE-SPARKY.2 for automated NMR-based protein structure research. Bioinformatics 34:1586–1588. https://doi.org/10.1093/bioinformatics/btx785
Puig S, Askeland E, Thiele DJ (2005) Coordinated remodeling of cellular metabolism during iron deficiency through targeted mRNA degradation. Cell 120:99–110. https://doi.org/10.1016/j.cell.2004.11.032
Stumpo DJ, Byrd NA, Phillips RS, Ghosh S, Maronpot RR, Castranio T, Meyers EN, Mishina Y, Blackshear PJ (2004) Chorioallantoic fusion defects and embryonic lethality resulting from disruption of Zfp36L1, a gene encoding a CCCH tandem zinc finger protein of the Tristetraprolin family. Mol Cell Biol 24:6445–6455. https://doi.org/10.1128/MCB.24.14.6445-6455.2004
Stumpo DJ, Broxmeyer HE, Ward T, Cooper S, Hangoc G, Chung YJ, Shelley WC, Richfield EK, Ray MK, Yoder MC, Aplan PD, Blackshear PJ (2009) Targeted disruption of Zfp36l2, encoding a CCCH tandem zinc finger RNA-binding protein, results in defective hematopoiesis. Blood 114:2401–2410. https://doi.org/10.1182/blood-2009-04-214619
Stumpo DJ, Trempus CS, Tucker CJ, Huang W, Li L, Kluckman K, Bortner DM, Blackshear PJ (2016) Deficiency of the placenta- and yolk sac-specific tristetraprolin family member ZFP36L3 identifies likely mRNA targets and an unexpected link to placental iron metabolism. Development 143:1424–1433. https://doi.org/10.1242/dev.130369
Taylor GA, Carballo E, Lee DM, Lai WS, Thompson MJ, Patel DD, Schenkman DI, Gilkeson GS, Broxmeyer HE, Haynes BF, Blackshear PJ (1996) A pathogenetic role for TNF alpha in the syndrome of cachexia, arthritis, and autoimmunity resulting from tristetraprolin (TTP) deficiency. Immunity 4:445–454. https://doi.org/10.1016/s1074-7613(00)80411-2
Venters RA, Coggins BE, Kojetin D, Cavanagh J, Zhou P (2005) (4,2)D projection–reconstruction experiments for protein backbone assignment: application to human carbonic anhydrase II and calbindin D(28K). J Am Chem Soc 127:8785–8795. https://doi.org/10.1021/ja0509580
Wells ML, Huang W, Li L, Gerrish KE, Fargo DC, Ozsolak F, Blackshear PJ (2012) Posttranscriptional regulation of cell-cell interaction protein-encoding transcripts by Zfs1p in Schizosaccharomyces pombe. Mol Cell Biol 32:4206–4214. https://doi.org/10.1128/MCB.00325-12
Wells ML, Hicks SN, Perera L, Blackshear PJ (2015) Functional equivalence of an evolutionarily conserved RNA binding module. J Biol Chem 290:24413–24423. https://doi.org/10.1074/jbc.M115.673012
Zimmerman DE, Kulikowski CA, Huang Y, Feng W, Tashiro M, Shimotakahara S, Chien C, Powers R, Montelione GT (1997) Automated analysis of protein NMR assignments using methods from artificial intelligence. J Mol Biol 269:592–610. https://doi.org/10.1006/jmbi.1997.1052
Acknowledgements
This work was supported by the Intramural Research Program of the National Institute of Environmental Health Sciences, NIH. We are grateful to the Duke NMR Spectroscopy Center for use of instrumentation, data analysis, and training, and to Dr. Leonard Spicer for advice and encouragement. Thanks also to Geoffrey Mueller and Robin Stanley for helpful comments on the manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
The authors declare that the experiments described herein comply with the current laws of the United States.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Hicks, S.N., Venters, R.A. & Blackshear, P.J. Backbone and sidechain 1H, 15N and 13C resonance assignments of the free and RNA-bound tandem zinc finger domain of the tristetraprolin family member from Selaginella moellendorffii. Biomol NMR Assign 16, 153–158 (2022). https://doi.org/10.1007/s12104-022-10073-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12104-022-10073-8