Abstract
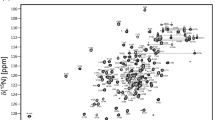
RbfA (ribosome binding factor A; 15.2 kDa) is a protein involved in ribosome biogenesis and has been shown to be important for growth at low temperatures and to act as a suppressor for a cold-sensitive mutation (C23U) in the ribosomal RNA of the small 30S ribosomal subunit. The 3D structure of isolated RbfA has been determined from several organisms showing that RbfA has type-II KH-domain fold topology similar to the KH domain of another assembly factor, Era, whose overexpression can compensate for the deletion of rbfA, suppressing both the cold sensitivity and abnormal accumulation of 17S rRNA in rbfA knockout stains. Interestingly, a RbfAΔ25 variant used in previous NMR studies, truncated at the C-terminal domain to remove 25 unstructured residues causing aggregation at room temperature, was biologically active in the sense that it could complement a knock-out of wildtype RbfA, although it did not act as a suppressor for a 16S cold-sensitive mutation (C23U), nor did it interact stably with the 30S subunit. To complement this work, we report the 1H, 13C, and 15 N backbone and sidechain NMR resonance assignments of full length RbfA from Escherichia coli measured under physiological conditions (pH 7.6). This construct contains seven additional C-terminal residues from the cloning (i.e. one alanine and six residues from the HRV 3C cleavage site) and no aggregation issues were observed over a 1-week period at 293 K. The assignment data has been deposited in the BMRB data bank under Accession No. 27857.


Similar content being viewed by others

Abbreviations
- IPTG:
-
Isopropyl-thio-β-D-galactoside
- E. coli :
-
Escherichia coli
- RbfA:
-
Ribosome binding factor A
- PIC:
-
Protease inhibitor cocktail
- TCEP:
-
Tris(2-carboxyethyl)-phosphine
- RCI:
-
Random coil index
References
Baba T, Ara T, Hasegawa M, Takai Y, Okumura Y, Baba M, Datsenko KA, Tomita M, Wanner BL, Mori H (2006) Construction of Escherichia coli K-12 in-frame, single-gene knockout mutants: the Keio collection. Mol Syst Biol 2:20000008
Berjanskii MV, Wishart DS (2005) A simple method to predict protein flexibility using secondary chemical shifts. J Am Chem Soc 127:14970–14971
Bylund GO, Wipemo LC, Lundberg LA, Wikström PM (1998) RimM and RbfA are essential for efficient processing of 16S rRNA in Escherichia coli. J Bacteriol 180:73–82
Chuang N-N, Huang C-C (2007) Interaction of integrin beta1 with cytokeratin 1 in neuroblastoma NMB7 cells. Biochem Soc Trans 35:1292–1294
Dammel CS, Noller HF (1995) Suppression of a cold-sensitive mutation in 16S rRNA by overexpression of a novel ribosome-binding factor, RbfA. Genes Dev 9:626–637
Datta PP, Wilson DN, Kawazoe M, Swami NK, Kaminishi T, Sharma MR, Booth TM, Takemoto C, Fucini P, Yokoyama S, Agrawal RK (2007) Structural aspects of RbfA action during small ribosomal subunit assembly. Mol Cell 28:434–445
Delaglio F, Grzesiek S, Vuister GW, Zhu G, Pfeifer J, Bax A (1995) NMRPipe: a multidimensional spectral processing system based on UNIX pipes. J Biomol NMR 6:277–293
Fristedt R, Scharff L, Clarke C, Wang Q, Lin C, Merchant S, Bock R (2015) A plant homolog of the bacterial ribosome-binding factor RbfA. Plant Physiol. 169:1419–1419
Geertsma ER, Dutzler R (2011) A versatile and efficient high-throughput cloning tool for structural biology. Biochemistry 50:3272–3278
Huang YJ, Swapna GVT, Rajan PK, Ke H, Xia B, Shukla K, Inouye M, Montelione GT (2003) Solution NMR structure of ribosome-binding factor A (RbfA), a cold-shock adaptation protein from Escherichia coli. J Mol Biol 327:521–536
Inoue K, Alsina J, Chen J, Inouye M (2003) Suppression of defective ribosome assembly in a rbfA deletion mutant by overexpression of Era, an essential GTPase in Escherichia coli. Mol Microbiol 48:1005–1016. https://doi.org/10.1046/j.1365-2958.2003.03475.x
Jones PG, Inouye M (1996) RbfA, a 30S ribosomal binding factor, is a cold-shock protein whose absence triggers the cold-shock response. Mol Microbiol 21:1207–1218
Lee W, Tonelli M, Markley JL (2015) NMRFAM-SPARKY: enhanced software for biomolecular NMR spectroscopy. Bioinformatics (Oxford, England) 31:1325–1327
Rozanska A, Richter-Dennerlein R, Rorbach J, Gao F, Lewis RJ, Chrzanowska-Lightowlers ZM, Lightowlers RN (2017) The human RNA-binding protein RBFA promotes the maturation of the mitochondrial ribosome. Biochem J 474:2145–2158
Shen Y, Delaglio F, Cornilescu G, Bax A (2009) TALOS+: a hybrid method for predicting protein backbone torsion angles from NMR chemical shifts. J Biomol NMR 44:213–223
Swapna GV, Shukla K, Huang YJ, Ke H, Xia B, Inouye M, Montelione GT (2001) Resonance assignments for cold-shock protein ribosome-binding factor A (RbfA) from Escherichia coli. J Biomol NMR 21:389–390
Xia B, Ke H, Shinde U, Inouye M (2003) The role of RbfA in 16S rRNA processing and cell growth at low temperature in Escherichia coli. J Mol Biol 332:575–584
Acknowledgements
This work was supported by grants from the European Union’s Seventh Framework Program (Marie Curie Actions; COFUND; to S.R.C. and A.S.), Marie Curie Action Career Integration Grant (PCIG14-GA-2013-632072 to P.F.), and Ministerio de Economía y Competitividad Grant (CTQ2017-82222-R to P.F., S.R.C.). We thank MINECO for the Severo Ochoa Excellence Accreditation (SEV-2016-0644) and the proteomics platform of CIC biogune for sample analysis by MALDI TOF mass spectroscopy.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Schedlbauer, A., Iturrioz, I., Ochoa-Lizarralde, B. et al. Backbone and sidechain NMR assignments for the ribosome maturation factor RbfA from Escherichia coli. Biomol NMR Assign 14, 317–321 (2020). https://doi.org/10.1007/s12104-020-09969-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12104-020-09969-0