Abstract
Objective
To investigate if expanded newborn screening using tandem mass spectroscopy (TMS) is adequate to detect low excretor phenotype in Indian Glutaric aciduria type I (GA-I) patients.
Methods
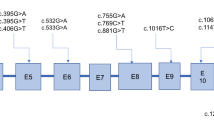
Ten GA-I patients were investigated for blood glutaryl carnitine (C5DC) levels on dried blood spot (DBS) by tandem mass spectroscopy and urine glutaric acid (GA) and 3-hydroxyglutaric acid (3-OH-GA) by gas chromatography-mass spectroscopy. The student’s T test and Pearson’s correlation were applied to draw a relationship between various biochemical parameters. Further confirmation of low excretors by DNA mutation analysis in the glutaryl CoA dehydrogenase (GCDH) gene was performed by polymerase chain reaction and Sangers sequencing.
Results
Among 10 GA-I patients, 7 patients were found to have high excretor, and 3 were found to have low excretor phenotype. The low excretors were found to have GCDH gene mutations. The mean C5DC levels in high and low excretors were 2.61 ± 2.02 μmol/L and 2.31 ± 1.00 μmol/L, respectively. In high excretors, C5DC levels correlated with GA (r = 0.95). In low excretors, C5DC levels correlated with 3-OH-GA (r = 0.99). No significant difference was found between C5DC levels of high and low excretors (p = 0.82).
Conclusions
The MS/MS, C5DC screening is a sensitive technique and detected 10 GA-I patients. Irrespective of the urine organic acid levels, Indian GA-I patients including low excretors seem to have a significantly elevated C5DC level and well above the stipulated cut-off values and therefore, expanded newborn screening is probably adequate to diagnose them.



Similar content being viewed by others
References
Barić I, Baraka K, Maradin M, et al. Glutaric aciduria type 1: an example of the importance of early detection of so-called cerebral organic aciduria. [Article in Croatian]. Lijec Vjesn. 2003;125:312–6.
Strauss KA, Puffenberger EG, Robinson DL, Morton DH. Type I glutaric aciduria, part 1: natural history of 77 patients. Am J Med Genet. 2003;121C:38–52.
Kölker S, Garbade SF, Greenberg CR, et al. Natural history, outcome, and treatment efficacy in children and adults with glutaryl-CoA dehydrogenase deficiency. Pediatr Res. 2006;59:840–7.
Vaidyanathan K, Narayanan MP, Vasudevan DM. Organic acidurias: an updated review. Indian J Clin Biochem. 2011;26:319–25.
Babu RP, Bishnupriya G, Thushara PK, et al. Detection of glutaric acidemia type 1 in infants through tandem mass spectrometry. Mol Genet Metabol Rep. 2015;3:75–9.
Fraidakis MJ, Liadinioti C, Stefanis L, et al. Rare late-onset presentation of glutaric aciduria type I in a 16-year-old woman with a novel GCDH mutation. JIMD Rep. 2015;18:85–92.
Busquets C, Merinero B, Christensen E, et al. Glutaryl-CoA dehydrogenase deficiency in Spain: evidence of two groups of patients, genetically, and biochemically distinct. Pediatr Res. 2000;48:315–22.
Boy N, Mengler K, Thimm E, et al. Newborn screening: a disease-changing intervention for glutaric aciduria type 1. Ann Neurol. 2018;83:970–9.
Hedlund GL, Longo N, Pasquali M. Glutaric acidemia type 1. Am J Med Genet P C Semin Med Genet. 2006;142C:86–94.
Kolker S, Christensen E, Leonard JV, et al. Diagnosis and management of glutaric aciduria type I-revised recommendations. J Inherit Metab Dis. 2011;34:677–94.
Boy N, Mühlhausen C, Maier EM, et al. Proposed recommendations for diagnosing and managing individuals with glutaric aciduria type I: second revision. J Inherit Metab Dis. 2017;40:75–101.
Heringer J, Boy N, Burgard P, Okun JG, Kolker S. Newborn screening for glutaric aciduria type I: benefits and limitations. Int J Neonatal Screen. 2015;1:57–68.
Horster F, Kolker S, Loeber JG, Cornel MC, Hoffmann GF, Burgard GF. Newborn screening programmes in europe, arguments and efforts regarding harmonisation: focus on organic acidurias. JIMD Rep. 2017;32:105–15.
Chace DH, Kalas TA, Naylor EW. Use of tandem mass spectrometry for multianalyte screening of dried blood specimens from newborns. Clin Chem. 2003;49:1797–817.
Heringer J, Boy SP, Ensenauer R, et al. Use of guidelines improves the neurological outcome in glutaric aciduria type I. Ann Neurol. 2010;68:743–52.
Wilcken B, Wiley V, Hammond J. Screening newborns for inborn errors of metabolism by tandem mass spectrometry. N Engl J Med. 2003;348:2304–12.
Lindner M, Kölker S, Schulze A, Christensen E, Greenberg CR, Hoffmann GF. Neonatal screening for glutaryl-CoA dehydrogenase deficiency. J Inherit Metab Dis. 2004;27:851–9.
Smith WE, Millington DS, Koeberl DK, Lesser PS. Glutaric acidemia, type I, missed by newborn screening in an infant with dystonia following promethazine administration. Pediatrics. 2001;107:1184–7.
Kaur G, Thakur K, Kataria S, et al. Current and future perspective of newborn screening: an Indian scenario. J Pediatr Endocrinol Metab. 2016;29:5–13.
Devi ARR, Naushad SM. Newborn screening in India. Indian J Pediatr. 2004;71:157–60.
Banta-Wright SA, Steiner RD. Tandem mass spectrometry in newborn screening: a primer for neonatal and perinatal nurses. J Perinat Neonatal Nurs. 2004;18:41–58.
Rakheja D, Jones VK, Burlina AB, Bennett MJ. Diagnosis of glutaric acidemia type I: a cautionary note. Lab Med. 2005;36:174–7.
Couce ML, López-Suárez O, Bóveda MD, et al. Glutaric aciduria type I: outcome of patients with early- versus late-diagnosis. Eur J Paediatr Neurol. 2013;17:383–9.
Radha Rama Devi A, Ramesh VA, Nagarajaram HA, Satish SP, Jayanthi U, Lingappa L. Spectrum of mutations in Glutaryl-CoA dehydrogenase gene in glutaric aciduria type I--study from South India. Brain Dev. 2016;38:54–60.
Gupta N, Singh PK, Kumar M, et al. Glutaric acidemia type 1-clinico-molecular profile and novel mutations in GCDH gene in Indian patients. JIMD Rep. 2015;21:45–55.
Flanagan JL, Simmons PA, Vehige J, Willcox MD, Garrett Q. Role of carnitine in disease. Nutr Metab. 2010;7:30.
Tortorelli S, Hahn SH, Cowan TM, Brewster TG, Rinaldo P, Matern D. The urinary excretion of glutarylcarnitine is an informative tool in the biochemical diagnosis of glutaric aciduria type I. Mol Genet Metab. 2005;84:137–43.
Keyser B, Glatzel M, Stellmer F, et al. Transport and distribution of 3-hydroxyglutaric acid before and during induced encephalopathic crises in a mouse model of glutaric aciduria type 1. Biochim Biophys Acta Mol Basis Dis. 2008;1782:385–90.
Al-Dirbashi OY, Kölker S, Ng D, et al. Diagnosis of glutaric aciduria type 1 by measuring 3-hydroxyglutaric acid in dried urine spots by liquid chromatography tandem mass spectrometry. J Inherit Metab Dis. 2011;34:173–80.
Author information
Authors and Affiliations
Contributions
All the authors have revised the manuscript and contributed to the drafting of the article. They confirm that the manuscript is an original version and has not been published in any other scientific journal or elsewhere; MS has performed the biochemical analysis and genetic analysis and drafted the manuscript. MK has been the referral physician for neonatal resuscitation and metabolic and nutritional management of the patient and has validated the results. KVTP and VAB have helped in cross checking the genetic reports and framing the script. All authors read and approved the final manuscript. Prof. Shyam Kumar Vootla, Professor and Chairman, Department of Biotechnology and Microbiology, Karnatak University, Dharwad, is the guarantor for this article.
Corresponding author
Ethics declarations
Conflict of Interest
None.
Source of Funding
Maulana Azad National Fellowship F1–17.1/2017–18/MANF-2017-18-KAR-75132.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Shaik, M., T. P., KV., Kamate, M. et al. Is Expanded Newborn Screening Adequate to Detect Indian Biochemical Low Excretor Phenotype Patients of Glutaric Aciduria Type I?. Indian J Pediatr 86, 995–1001 (2019). https://doi.org/10.1007/s12098-019-03017-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12098-019-03017-z