Abstract
Objectives
Glucose-6-phosphate isomerase (GPI) deficiency is an autosomal recessive genetic disorder causing hereditary non-spherocytic hemolytic anemia (HNSHA) coupled with a neurological disorder. The aim of this study was to identify GPI genetic defects in a cohort of Indian patients with HNSHA coupled with neurological dysfunction.
Methods
Thirty-five patients were screened for GPI deficiency in the HNSHA patient group; some were having neurological dysfunction. Enzyme activity was measured by spectrophotometric method. The genetic study was done by single-stranded conformation polymorphism (SSCP) analysis, restriction fragment length polymorphism (RFLP) analysis by the restriction enzyme AciI for p.Arg347His (p.R347H) and confirmation by Sanger’s sequencing.
Results
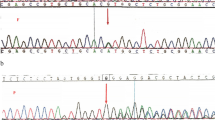
Out of 35 patients, 15 showed 35% to 70% loss of GPI activity, leading to neurological problems with HNSHA. Genetic analysis of PCR products of exon 12 of the GPI gene showed altered mobility on SSCP gel. Sanger’s sequencing revealed a homozygous c1040G > A mutation predicting a p.Arg347His replacement which abolishes AciI restriction site. The molecular modeling analysis suggests p.Arg347 is involved in dimerization of the enzyme. Also, this mutation generates a more labile enzyme which alters its three-dimensional structure and function.
Conclusions
This report describes the high prevalence of p.Arg347His pathogenic variant identified in Indian GPI deficient patients with hemolytic anemia and neuromuscular impairment. It suggests that neuromuscular impairment with hemolytic anemia cases could be investigated for p.Arg347His pathogenic variant causing GPI deficiency because of neuroleukin activity present in the GPI monomer which has neuroleukin action at the same active site and generates neuromuscular problems as well as hemolytic anemia.



Similar content being viewed by others
References
Manco L, Bento C, Victor BL, et al. Hereditary nonspherocytic hemolytic anemia caused by red cell glucose-6-phosphate isomerase (GPI) deficiency in two Portuguese patients: clinical features and molecular study. Blood Cells Mol Dis. 2016;60:18–23.
Kugler W, Breme K, Laspe P, et al. Molecular basis of neurological dysfunction coupled with hemolytic anemia in human glucose-6-phosphate isomerase (GPI) deficiency. Hum Genet. 1998;103:450–4.
Haga A, Niinaka Y, Raz A. Phosphohexose isomerase/autocrine motility factor/neuroleukin/maturation factor is a multifunctional phosphoprotein. Biochim Biophys Acta. 2000;1480:235–44.
Ravindranath Y, Paglia DE, Warrier I, Valentine W, Nakatani M, Brockway RA. Glucose phosphate isomerase deficiency as a cause of hydrops fetalis. N Engl J Med. 1987;316:258–61.
Kanno H, Fujii H, Hirono A, et al. Molecular analysis of glucose phosphate Isomerase deficiency associated with hereditary hemolytic anemia. Blood. 1996;88:2321–5.
Warang P, Kedar P, Ghosh K, Colah RB. Hereditary non-spherocytic hemolytic anemia and severe glucose phosphate isomerase deficiency in an Indian patient homozygous for the L487F mutation in the human GPI gene. Int J Hematol. 2012;96:263–7.
Zaidi AU, Kedar P, Koduri PR, et al. Glucose phosphate isomerase (GPI) Tadikonda: characterization of a novel Pro340Ser mutation. Pediatr Hematol Oncol. 2017;34:449–54.
Kedar P, Gupta V, Dongerdiye R, Chiddarwar A, Warang P, Madkaikar MR. Molecular diagnosis of unexplained hemolytic anemia using targeted next-generation sequencing panel revealed (p.Ala337Thr) novel mutation in the GPI gene in two Indian patients. J Clin Pathol. 2019;72:81–5.
Walker JI, Layton DM, Bellingham AJ, Morgan MJ, Faik P. DNA sequence abnormalities in human glucose 6-phosphate isomerase deficiency. Hum Mol Genet. 1993;2:327–9.
Kugler W, Lakomek M. Glucose-6-phosphate isomerase deficiency. Best Pract Res Clin Haematol. 2000;13:89–101.
Beutler E, West C, Britton HA, Harris J, Forman L. Glucose phosphate isomerase (GPI) deficiency mutations associated with hereditary nonspherocytic hemolytic anemia (HNSHA). Blood Cells Mol Dis. 1997;23:402–9.
Xu W, Beutler E. The characterization of gene mutations for human glucose phosphate isomerase deficiency associated with chronic hemolytic anemia. J Clin Invest. 1994;94:2326–9.
Baronciani L, Zanella A, Bianchi P, et al. Study of the molecular defects in glucose phosphate isomerase-deficient patients affected by chronic hemolytic anemia. Blood. 1996;88:2306–10.
Repiso A, Oliva B, Vives-Corrons JL, Beutler E, Carreras J, Climent F. Red cell glucose phosphate isomerase (GPI): a molecular study of three novel mutations associated with hereditary nonspherocytic hemolytic anemia. Hum Mutat. 2006;27:1159.
Clarke JL, Vulliamy TJ, Roper D. Combined glucose-6-phosphate dehydrogenase, and glucose phosphate isomerase deficiency can alter clinical outcome. Blood Cells Mol Dis. 2003;30:258–63.
Lakomek M, Winkler H. Erythrocyte pyruvate kinase- and glucose phosphate isomerase deficiency: perturbation of glycolysis by structural defects and functional alterations of defective enzymes and its relation to the clinical severity of chronic hemolytic anemia. Biophys Chem. 1997;66:269–84.
Mojzikova R, Koralkova P, Holub D, et al. Two novel mutations (p.(Ser160Pro) and p.(Arg472Cys)) causing glucose-6-phosphate isomerase deficiency are associated with erythroid dysplasia and inappropriately suppressed hepcidin. Blood Cells Mol Dis. 2018;69:23–9.
Burger NCM, Van Wijk R, Bresters D, Schell EA. A novel mutation of glucose phosphate isomerase (GPI) causing severe neonatal anemia due to GPI deficiency. J Pediatr Hematol Oncol. 2018 Dec 21. [Ahead of print].
Zhu X, Petrovski S, Xie P, et al. Whole-exome sequencing in undiagnosed genetic diseases: interpreting 119 trios. Genet Med. 2015;17:774–81.
Dacie JV, Lewis SM. Practical Haematology. 10th ed. Edinburgh: Churchill Livingstone; 2006. p. 60–78.
Beutler E. Red Cell Metabolism: A Manual of Biochemical Methods. 3rd ed. New York: Grune & Stratton, Inc; 1984.
Davies C, Muirhead H, Chirgwin J. The structure of human phosphoglucose isomerase complexed with a transition-state analog. Acta Crystallogr D Biol Crystallogr. 2003;59:1111–3.
Feyfant E, Sali A, Fiser A. Modeling mutations in protein structures. Protein Sci. 2007;16:2030–41.
Repiso A, Oliva B, Vives Corrons JL, Carreras J, Climent F. Glucose phosphate isomerase deficiency: enzymatic and familial characterization of Arg346His mutation. Biochim Biophys Acta. 2005;1740:467–71.
Jamwal M, Aggarwal A, Das A, et al. Next-generation sequencing unravels homozygous mutation in glucose-6-phosphate isomerase, GPIc.1040G> A (p.Arg347His) causing hemolysis in an Indian infant. Clin Chim Acta. 2017;468:81–4.
Magor GW, Tallack MR, Gillinder KR, et al. KLF1-null neonates display hydrops fetalis and a deranged erythroid transcriptome. Blood. 2015;125:2405–17.
Koralkova P, van Solinge WW, van Wijk R. Rare hereditary red blood cell enzymopathies associated with hemolytic anemia pathophysiology, clinical aspects, and laboratory diagnosis. Int J Lab Hematol. 2014;36:388–97.
Grace RF, Zanella A, Neufeld EJ, et al. Erythrocyte pyruvate kinase deficiency: 2015 status report. Am J Hematol. 2015;90:825–30.
Shalev O, Shalev RS, Forman L, Beutler E. GPI mount scopus--a variant of glucose phosphate isomerase deficiency. Ann Hematol. 1993;67:197–200.
Schröter W, Eber SW, Bardosi A, Gahr M, Gabriel M, Sitzmann FC. Generalized glucose phosphate Isomerase (GPI) deficiency causing hemolytic anemia, neuromuscular symptoms and impairment of granulocytic function: a new syndrome due to a new stable GPI variant with a diminished specific activity (GPI Homburg). Eur J Pediatr. 1985;144:301–5.
Roy NB, Wilson EA, Henderson S, et al. A novel 33-gene-targeted resequencing panel provides accurate, clinical-grade diagnosis and improves patient management for rarely inherited anemias. Br J Haematol. 2016;175:318–30.
Neubauer BA, Eber SW, Lakomek M, Gahr M, Schröter W. Combination of congenital nonspherocytic hemolytic anemia and impairment of granulocyte function in severe glucose phosphate isomerse deficiency. A new variant enzyme designated GPI Calden. Acta Haematol. 1990;83:206–10.
Author information
Authors and Affiliations
Contributions
PSK: Designed the experiment. RMD, AC, RD, PC, VG: Collected the biochemical, hematological data and performed lab experiments. HP, AS, SB, SC: Referred the case and did clinical examinations. PSK, PW, RD: Conducted the literature search, designed the figures and tables, and analyzed and interpreted the data. PSK, RD: Revised the first draft and completed the final manuscript. All authors approved the final manuscript. PC, VG, RD, AC, PW, PSK prepared the manuscript. MM is the Director, NIIH and also a clinician. She gave her suggestion about the cases and helped in clinical correlation; All authors read and approved the final manuscript. PSK is the guarantor.
Corresponding author
Ethics declarations
Conflict of Interest
None.
Source of Funding
This study was performed with the financial support from Indian Council of Medical Research, New Delhi, and Department of Biotechnology, New Delhi.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Kedar, P.S., Dongerdiye, R., Chilwirwar, P. et al. Glucose Phosphate Isomerase Deficiency: High Prevalence of p.Arg347His Mutation in Indian Population Associated with Severe Hereditary Non-Spherocytic Hemolytic Anemia Coupled with Neurological Dysfunction. Indian J Pediatr 86, 692–699 (2019). https://doi.org/10.1007/s12098-019-02928-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12098-019-02928-1