Abstract
Purpose
Cytokines are vital pro-inflammatory factors and involved in tumor immune infiltration, and immune infiltration is closely related to PD-1/PD-L1 blockades immunotherapy. This study aims to explore the associations between cytokines and prognosis and also PD-1/PD-L1 expression in early lung adenocarcinoma, which is seldom reported.
Methods
324 early lung adenocarcinoma patients with prior surgical resection were included and the associations between overall survival time and clinical factors and also cytokines including IL-1β, IL-6 and TNF-α were analyzed by multivariate cox regression and Kaplan–Meier curve (log-rank test). Resected tumor samples were randomly obtained to detect the PD-1/PD-L1 expression by immunohistochemistry, and Chi square test was used for relations between cytokines and PD-1/PD-L1 expression.
Results
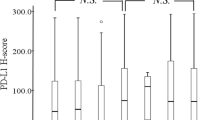
In this study group, 26.2% patients showed a high level of IL-1β and patients with high IL-1β level showed 19 months shortened mOS than those with normal IL-1 β expression (mOS: 24.00, 95%CI 11.98–36.02 vs 43.00, 95% CI 37.37–48.63, p = 0.017). Among detected samples, the positive rate of PD-1 was 25.0% (13/52), and the positive rate of PD-L1 was 37.3% (19/52). The positive rate of PD-1 was 36.1% higher in high-IL-1 β-level group as compared to normal-IL-1β-level group (50.0% vs 13.9%, p = 0.012). No significant association was found between IL-1 β and PD-L1 expression.
Conclusion
High expression level of IL-1β was correlated with poor prognosis and higher positive rate of PD-1 expression, which gave us insights into biomarkers of survival prediction and immunotherapy in lung adenocarcinoma. Further studies were still needed.




Similar content being viewed by others
Data availability
All data generated or analyzed during this study are included in this published article.
Code availability
No code was used in this study.
References
Siegel RL, Miller KD, Jemal A. Cancer statistics, 2020. CA: Cancer J Clin. 2020;70(1):7–30. https://doi.org/10.3322/caac.21590.
Herbst RS, Morgensztern D, Boshoff C. The biology and management of non-small cell lung cancer. Nature. 2018;553(7689):446–54. https://doi.org/10.1038/nature25183.
Travis WD, Brambilla E, Burke AP, Marx A, Nicholson AG. Introduction to The 2015 World Health Organization classification of tumors of the lung, pleura, thymus, and heart. J Thorac Oncol: Off Pub Int Assoc Study Lung Cancer. 2015;10(9):1240–2. https://doi.org/10.1097/jto.0000000000000663.
Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144(5):646–74. https://doi.org/10.1016/j.cell.2011.02.013.
Quail DF, Joyce JA. Microenvironmental regulation of tumor progression and metastasis. Nat Med. 2013;19(11):1423–37. https://doi.org/10.1038/nm.3394.
Palucka AK, Coussens LM. The basis of oncoimmunology. Cell. 2016;164(6):1233–47. https://doi.org/10.1016/j.cell.2016.01.049.
Oppenheim JJ. The future of the cytokine discipline. Cold Spring Harb Perspect Biol. 2018;10(9):a028498. https://doi.org/10.1101/cshperspect.a028498.
Orditura M, Romano C, De Vita F, Galizia G, Lieto E, Infusino S, et al. Behaviour of interleukin-2 serum levels in advanced non-small-cell lung cancer patients: relationship with response to therapy and survival. Cancer Immunol Immunother. 2000;49(10):530–6. https://doi.org/10.1007/s002620000150.
Songür N, Kuru B, Kalkan F, Ozdilekcan C, Cakmak H, Hizel N. Serum interleukin-6 levels correlate with malnutrition and survival in patients with advanced non-small cell lung cancer. Tumori. 2004;90(2):196–200.
Ding X, Zhang J, Liu D, Xu W, Lu DY, Zhang LP, et al. Serum expression level of IL-6 at the diagnosis time contributes to the long-term prognosis of SCLC patients. J Cancer. 2018;9(5):792–6. https://doi.org/10.7150/jca.22656.
Waldmann TA. Cytokines in cancer immunotherapy. Cold Spring Harb Perspect Biol. 2018;10(12):a028472. https://doi.org/10.1101/cshperspect.a028472.
Rosenberg SA, Lotze MT, Yang JC, Aebersold PM, Linehan WM, Seipp CA, et al. Experience with the use of high-dose interleukin-2 in the treatment of 652 cancer patients. Ann Surg. 1989;210(4):474–85. https://doi.org/10.1097/00000658-198910000-00008.
Fyfe G, Fisher RI, Rosenberg SA, Sznol M, Parkinson DR, Louie AC. Results of treatment of 255 patients with metastatic renal cell carcinoma who received high-dose recombinant interleukin-2 therapy. J Clin Oncol: Off J Am Soc Clin Oncol. 1995;13(3):688–96. https://doi.org/10.1200/JCO.1995.13.3.688.
Atkins MB, Lotze MT, Dutcher JP, Fisher RI, Weiss G, Margolin K, et al. High-dose recombinant interleukin 2 therapy for patients with metastatic melanoma: analysis of 270 patients treated between 1985 and 1993. J Clin Oncol: Off J Am Soc Clin Oncol. 1999;17(7):2105–16. https://doi.org/10.1200/JCO.1999.17.7.2105.
Gutterman JU, Blumenschein GR, Alexanian R, Yap HY, Buzdar AU, Cabanillas F, et al. Leukocyte interferon-induced tumor regression in human metastatic breast cancer, multiple myeloma, and malignant lymphoma. Ann Intern Med. 1980;93(3):399–406. https://doi.org/10.7326/0003-4819-93-3-399.
Amato R. Modest effect of interferon alfa on metastatic renal-cell carcinoma. Lancet (London, England). 1999;353(9146):6–7. https://doi.org/10.1016/S0140-6736(05)74876-5.
Ridker PM, MacFadyen JG, Thuren T, Everett BM, Libby P, Glynn RJ, et al. Effect of interleukin-1β inhibition with canakinumab on incident lung cancer in patients with atherosclerosis: exploratory results from a randomised, double-blind, placebo-controlled trial. Lancet (London, England). 2017;390(10105):1833–42. https://doi.org/10.1016/S0140-6736(17)32247-X.
Sharma P, Allison JP. The future of immune checkpoint therapy. Science (New York, NY). 2015;348(6230):56–61. https://doi.org/10.1126/science.aaa8172.
Herbst RS, Baas P, Kim D-W, Felip E, Pérez-Gracia JL, Han J-Y, et al. Pembrolizumab versus docetaxel for previously treated, PD-L1-positive, advanced non-small-cell lung cancer (KEYNOTE-010): a randomised controlled trial. Lancet (London, England). 2016;387(10027):1540–50. https://doi.org/10.1016/S0140-6736(15)01281-7.
Reck M, Brahmer JR. Pembrolizumab in non-small-cell lung cancer. N Engl J Med. 2017;376(10):997. https://doi.org/10.1056/nejmc1615559.
Akbay EA, Koyama S, Carretero J, Altabef A, Tchaicha JH, Christensen CL, et al. Activation of the PD-1 pathway contributes to immune escape in EGFR-driven lung tumors. Cancer Discov. 2013;3(12):1355–63. https://doi.org/10.1158/2159-8290.CD-13-0310.
Ota K, Azuma K, Kawahara A, Hattori S, Iwama E, Tanizaki J, et al. Induction of PD-L1 expression by the EML4-ALK oncoprotein and downstream signaling pathways in non-small cell lung cancer. Clin Cancer Res: Off J Am Assoc Cancer Res. 2015;21(17):4014–21. https://doi.org/10.1158/1078-0432.CCR-15-0016.
Netea MG, Balkwill F, Chonchol M, Cominelli F, Donath MY, Giamarellos-Bourboulis EJ, et al. A guiding map for inflammation. Nat Immunol. 2017;18(8):826–31. https://doi.org/10.1038/ni.3790.
Dinarello CA, Simon A, van der Meer JWM. Treating inflammation by blocking interleukin-1 in a broad spectrum of diseases. Nat Rev Drug Discov. 2012;11(8):633–52. https://doi.org/10.1038/nrd3800.
Mantovani A, Barajon I, Garlanda C. IL-1 and IL-1 regulatory pathways in cancer progression and therapy. Immunol Rev. 2018;281(1):57–61. https://doi.org/10.1111/imr.12614.
Voronov E, Shouval DS, Krelin Y, Cagnano E, Benharroch D, Iwakura Y, et al. IL-1 is required for tumor invasiveness and angiogenesis. Proc Natl Acad Sci USA. 2003;100(5):2645–50. https://doi.org/10.1073/pnas.0437939100.
Apte RN, Dotan S, Elkabets M, White MR, Reich E, Carmi Y, et al. The involvement of IL-1 in tumorigenesis, tumor invasiveness, metastasis and tumor-host interactions. Cancer Metastasis Rev. 2006;25(3):387–408. https://doi.org/10.1007/s10555-006-9004-4.
Krelin Y, Voronov E, Dotan S, Elkabets M, Reich E, Fogel M, et al. Interleukin-1beta-driven inflammation promotes the development and invasiveness of chemical carcinogen-induced tumors. Cancer Res. 2007;67(3):1062–71. https://doi.org/10.1158/0008-5472.CAN-06-2956.
Giavazzi R, Garofalo A, Bani MR, Abbate M, Ghezzi P, Boraschi D, et al. Interleukin 1-induced augmentation of experimental metastases from a human melanoma in nude mice. Cancer Res. 1990;50(15):4771–5.
Ting JPY, Duncan JA, Lei Y. How the noninflammasome NLRs function in the innate immune system. Science (New York, NY). 2010;327(5963):286–90. https://doi.org/10.1126/science.1184004.
van de Veerdonk FL, Netea MG, Dinarello CA, Joosten LAB. Inflammasome activation and IL-1β and IL-18 processing during infection. Trends Immunol. 2011;32(3):110–6. https://doi.org/10.1016/j.it.2011.01.003.
Kim B-H, Chee JD, Bradfield CJ, Park E-S, Kumar P, MacMicking JD. Interferon-induced guanylate-binding proteins in inflammasome activation and host defense. Nat Immunol. 2016;17(5):481–9. https://doi.org/10.1038/ni.3440.
Cai S, Batra S, Wakamatsu N, Pacher P, Jeyaseelan S. NLRC4 inflammasome-mediated production of IL-1β modulates mucosal immunity in the lung against gram-negative bacterial infection. J Immunol. 2012;188(11):5623–35. https://doi.org/10.4049/jimmunol.1200195.
Nishimura H, Nose M, Hiai H, Minato N, Honjo T. Development of lupus-like autoimmune diseases by disruption of the PD-1 gene encoding an ITIM motif-carrying immunoreceptor. Immunity. 1999;11(2):141–51. https://doi.org/10.1016/s1074-7613(00)80089-8.
Freeman GJ, Long AJ, Iwai Y, Bourque K, Chernova T, Nishimura H, et al. Engagement of the PD-1 immunoinhibitory receptor by a novel B7 family member leads to negative regulation of lymphocyte activation. J Exp Med. 2000;192(7):1027–34. https://doi.org/10.1084/jem.192.7.1027.
Mantovani A, Marchesi F, Malesci A, Laghi L, Allavena P. Tumour-associated macrophages as treatment targets in oncology. Nat Rev Clin Oncol. 2017;14(7):399–416. https://doi.org/10.1038/nrclinonc.2016.217.
Rőszer T. Understanding the mysterious M2 macrophage through activation markers and effector mechanisms. Mediat Inflamm. 2015;2015:816460. https://doi.org/10.1155/2015/816460.
Tang X. Tumor-associated macrophages as potential diagnostic and prognostic biomarkers in breast cancer. Cancer Lett. 2013;332(1):3–10. https://doi.org/10.1016/j.canlet.2013.01.024.
Gordon SR, Maute RL, Dulken BW, Hutter G, George BM, McCracken MN, et al. PD-1 expression by tumour-associated macrophages inhibits phagocytosis and tumour immunity. Nature. 2017;545(7655):495–9. https://doi.org/10.1038/nature22396.
Tarhini AA, Cherian J, Moschos SJ, Tawbi HA, Shuai Y, Gooding WE, et al. Safety and efficacy of combination immunotherapy with interferon alfa-2b and tremelimumab in patients with stage IV melanoma. J Clin Oncol: Off J Am Soc Clin Oncol. 2012;30(3):322–8. https://doi.org/10.1200/JCO.2011.37.5394.
Yu P, Steel JC, Zhang M, Morris JC, Waldmann TA. Simultaneous blockade of multiple immune system inhibitory checkpoints enhances antitumor activity mediated by interleukin-15 in a murine metastatic colon carcinoma model. Clin Cancer Res: Off J Am Assoc Cancer Res. 2010;16(24):6019–28. https://doi.org/10.1158/1078-0432.ccr-10-1966.
Yu P, Steel JC, Zhang M, Morris JC, Waitz R, Fasso M, et al. Simultaneous inhibition of two regulatory T-cell subsets enhanced Interleukin-15 efficacy in a prostate tumor model. Proc Natl Acad Sci USA. 2012;109(16):6187–92. https://doi.org/10.1073/pnas.1203479109.
Funding
This work was supported by the National Natural Science Foundation of China (No. 81572269, No. 81672279), the Science and Technology Commission of Shanghai Municipality (No. 17411969100).
Author information
Authors and Affiliations
Contributions
BS and KA designed and coordinated this study. XD analyzed the data and drafted the manuscript, JZ and MS collected the cases and recorded the information, DL and RZ finished the follow-up and analyzed part of the data, LZ was responsible for pathology-related testing. All authors approved the final manuscript.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
This study was conducted under the approval of the appropriate Ethics Committees in Shanghai Pulmonary Hospital.
Informed consent
Written informed consent was obtained for each participant.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Ding, X., Zhang, J., Shi, M. et al. High expression level of interleukin-1β is correlated with poor prognosis and PD-1 expression in patients with lung adenocarcinoma. Clin Transl Oncol 23, 35–42 (2021). https://doi.org/10.1007/s12094-020-02392-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12094-020-02392-w