Abstract
Background
Response to chemotherapy is a prognostic factor in patients with Ewing sarcoma (ES); the role of FDG PET to predict response in these patients has not been thoroughly investigated. We evaluated the diagnostic accuracy and the potential of FDG PET to predict response to chemotherapy (CHT).
Materials and methods
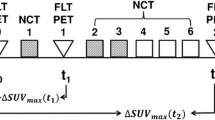
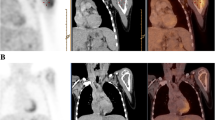
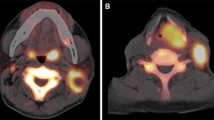
We analyzed data of 50 patients with ES (median age 12.6 years). All patients were treated with neoadjuvant CHT, and underwent surgery for local control. All patients had 18F-FDG PET/CT at diagnosis and after induction CHT, prior to local control. We compared response assessed by histopathology with FDG PET using standard uptake values (SUVs).
Results
Median SUV at diagnosis (SUV I) was 5 (range 1.2–17), and median SUV after neoadjuvant chemotherapy (SUV II) was 1.8 (range 0–8.4). Median SUV II/I ratio was 0.3 (range 0–1). SUV at diagnosis was significantly lower in patients with good histological response than in patients with poor histological response (median 3.8 vs. 7.2, p 0.02). We found a significant correlation between SUV II and outcome; the positive predictive value of an SUV II ≤ 2.5 for favorable response was 84.21 %, and the median SUV II was significantly higher in patients with disease progression (2.3 vs. 1.6, p = 0.04). In multivariate analysis, necrosis and SUV II were significant predictors of outcome.
Conclusions
18F-FDG PET demonstrates high diagnostic accuracy for response to initial chemotherapy in patients with ES and it correlates with outcome. The role of FDG PET in predicting response and outcome should be further investigated.

Similar content being viewed by others
References
Rodriguez-Galindo C, Liu T, Krasin MJ, Wu J, Billups CA, Daw NC, et al. Analysis of prognostic factors in Ewing sarcoma family of tumors: review of St.Jude Children’s Research Hospital studies. Cancer. 2007;110(2):375–84.
Rodriguez-Galindo C, Billups CA, Kun LE, Rao BN, Pratt CB, Merchant TE, et al. Survival after recurrence of Ewing tumors. Cancer. 2002;94(2):561–9.
Raciborska A, Bilska K, Drabko K, Chaber R, Sobol G, Pogorzała M, et al. Validation of a multimodal treatment protocol for Ewing sarcoma—a report from the Polish Pediatric Oncology Group. Pediatr Blood Cancer. 2014;61(12):2170–4.
Ladenstein R, Pötschger U, Le Deley MC, Whelan J, Paulussen M, Oberlin O, et al. Primary disseminated multifocal Ewing sarcoma: results of the Euro-EWING 99 trial. J Clin Oncol. 2010;28:3284–91.
Rodriguez-Galindo C, Navid F, Liu T, Billups CA, Rao BN, Krasin MJ. Prognostic factors for local and distant control in Ewing sarcoma family of tumors. Ann Oncol. 2008;19(4):814–20.
Shankar AG, Ashley S, Craft AW, Pinkerton CR. Outcome after relapse in an unselected cohort of children and adolescents with Ewing sarcoma. Med Pediatr Oncol. 2003;40:141–7.
Raciborska A, Bilska K, Drabko K, Chaber R, Pogorzala M, Wyrobek E, et al. Vincristine, irinotecan and temozolomide in patients with refractory Ewing sarcoma. Pediatr Blood Cancer. 2013;60(10):1621–5.
Jurgens C, Weston C, Lewis I, Whelan J, Paulussen M, Oberlin O, et al. Safety assessment of intensive induction with vincristine, ifosfamide, doxorubicin, and etoposide (VIDE) in the treatment of Ewing tumors in the EURO-E.W.I.N.G. 99 clinical trial. Pediatr Blood Cancer. 2006;47(1):22–9.
Le Deley MC, Paulussen M, Lewis I, Brennan B, Ranft A, Whelan J, et al. Cyclophosphamide compared with ifosfamide in consolidation treatment of standard-risk Ewing sarcoma: results of the randomized noninferiority Euro-EWING99-R1 trial. J Clin Oncol. 2014;32(23):2440–8.
Paulussen M, Craft AW, Lewis I, Hackshaw A, Douglas C, Dunst J, et al. Results of the EICESS-92 Study: two randomized trials of Ewing’s sarcoma treatment—cyclophosphamide compared with ifosfamide in standard-risk patients and assessment of benefit of etoposide added to standard treatment in high-risk patients. J Clin Oncol. 2008;26(27):4385–93.
Leavey PJ, Mascarenhas L, Marina N, Chen Z, Krailo M, Miser J, et al. Prognostic factors for patients with Ewing sarcoma (EWS) at first recurrence following multi-modality therapy: a report from the Children’s Oncology Group. Pediatr Blood Cancer. 2008;51:334–8.
Biswas B, Rastogi S, Khan SA, Mohanti BK, Sharma DN, Sharma MC, et al. Outcomes and prognostic factors for Ewing-family tumors of the extremities. J Bone Joint Surg Am. 2014;96(10):841–9.
Franzius C, Sciuk J, Brinkschmidt C, Jürgens H, Schober O. Evaluation of chemotherapy response in primary bone tumors with F-18 FDG positron emission tomography compared with histologically assessed tumor necrosis. Clin Nucl Med. 2000;25(11):874–81.
Treglia G, Salsano M, Stefanelli A, Mattoli MV, Giordano A, Bonomo L. Diagnostic accuracy of 18F-FDG-PET and PET/CT in patients with Ewing sarcoma family tumours: a systematic review and a meta-analysis. Skeletal Radiol. 2012;41(3):249–56.
Gerth HU, Juergens KU, Dirksen U, Gerss J, Schober O, Franzius C. Significant benefit of multimodal imaging: PET/CT compared with PET alone in staging and follow-up of patients with Ewing tumors. J Nucl Med. 2007;48(12):1932–9.
Furth C, Amthauer H, Denecke T, Ruf J, Henze G, Gutberlet M. Impact of whole-body MRI and FDG-PET on staging and assessment of therapy response in a patient with Ewing sarcoma. Pediatr Blood Cancer. 2006;47(5):607–11.
Newman EN, Jones RL, Hawkins DS. An evaluation of [F-18]-fluorodeoxy-d-glucose positron emission tomography, bone scan, and bone marrow aspiration/biopsy as staging investigations in Ewing sarcoma. Pediatr Blood Cancer. 2013;60(7):1113–7.
Völker T, Denecke T, Steffen I, Misch D, Schönberger S, Plotkin M, et al. Positron emission tomography for staging of pediatric sarcoma patients: results of a prospective multicenter trial. J Clin Oncol. 2007;25(34):5435–41.
Gyorke T, Zajic T, Lange A, Schäfer O, Moser E, Makó E, et al. Impact of FDG PET staging of Ewing sarcoma and primitive neuroectodermal tumors. Nucl Med Commun. 2006;27:17–24.
Franzius C, Sciuk J, Daldrup-Link HE, Jürgens H, Schober O. FDG-PET for detection of osseous metastases from malignant primary bone tumours: comparison with bone scintigraphy. Eur J Nucl Med. 2000;27(9):1305–11.
Sharma P, Khangembam BC, Suman KC, Singh H, Rastogi S, Khan SA, et al. Diagnostic accuracy of 18F-FDP PET/CT detecting recurrence in patients with primary skeletal Ewing sarcoma. Eur J Nucl Med Mol Imaging. 2013;40(7):1036–43.
Mody RJ, Bui C, Hutchinson RJ, Yanik GA, Castle VP, Frey KA, et al. FDG PET imaging of childhood sarcomas. Pediatr Blood Cancer. 2010;54(2):222–7.
Pieper S, Ranft A, Braun-Munzinger G, Jurgens H, Paulussen M, Dirksen U. Ewing’s Tumors over the age of 40–a retrospective analysis of 47 patients treated according to the international clinical trials EICESS 92 and EURO-E.W.I.N.G. 99. Onkologie. 2008;31:657–63.
Stauss J, Franzius C, Pfluger T, Juergens KU, Biassoni L, Begent J, et al. Guidelines for 18F-FDG PET and PET-CT imaging in paediatric oncology. Eur J Nucl Med Mol Imaging. 2008;35(8):1581–8.
Hawkins DS, Schuetze SM, Butrynski JE, Rajendran JG, Vernon CB, Conrad EU 3rd, et al. [18F] Fluorodeoxyglucose positron emission tomography predicts outcome for Ewing sarcoma family of tumors. J Clin Oncol. 2005;23(34):8828–34.
Hawkins DS, Rajendran JG, Conrad EU 3rd, Bruckner JD, Eary JF. Evaluation of chemotherapy response in pediatric bone sarcomas by [18F]-Fluorodeoxy-d-Glucose positron emission tomography. Cancer. 2002;94(12):3277–84.
Brisse H, Ollivier L, Edeline V, Pacquement H, Michon J, Glorion C, et al. Imaging of malignant tumours of the long bones in children: monitoring response to neoadjuvant chemotherapy and preoperative assessment. Pediatr Radiol. 2004;34(8):595–605.
Gupta K, Pawaskar A, Basu S, Rajan MG, Asopa RV, Arora B, et al. Potential role of FDG PET imaging in predicting metastatic potential and assessment of therapeutic response to neoadjuvant chemotherapy in Ewing sarcoma family of tumors. Clin Nucl Med. 2011;36(11):973–7.
Gaston LL, Di Bella C, Slavin J, Hicks RJ, Choong PF. 18F-FDG PET response to neoadjuvant chemotherapy for Ewing sarcoma and osteosarcoma are different. Skeletal Radiol. 2011;40(8):1007–15.
Iagaru A, Chwala S, Menendez L, Conti PS. 18F-FDG PET and PET/CT for detection of pulmonary metastases from musculoskeletal sarcomas. Nucl Med Commun. 2006;27:795–802.
Acknowledgments
Special thanks to the Children’s Medical Care Foundation, and Mr Bjoern Martinoff, President for the international support.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Nothing to declare.
Rights and permissions
About this article
Cite this article
Raciborska, A., Bilska, K., Drabko, K. et al. Response to chemotherapy estimates by FDG PET is an important prognostic factor in patients with Ewing sarcoma. Clin Transl Oncol 18, 189–195 (2016). https://doi.org/10.1007/s12094-015-1351-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12094-015-1351-6