Abstract
One of the main challenges in ecology is to determine the cause of population fluctuations. Both theoretical and empirical studies suggest that delayed density dependence instigates cyclic behavior in many populations; however, underlying mechanisms through which this occurs are often difficult to determine and may vary within species. In this paper, we consider single species population dynamics affected by the Allee effect coupled with discrete time delay. We use two different mathematical formulations of the Allee effect and analyze (both analytically and numerically) the role of time delay in different feedback mechanisms such as competition and cooperation. The bifurcation value of the delay (that results in the Hopf bifurcation) as a function of the strength of the Allee effect is obtained analytically. Interestingly, depending on the chosen delayed mechanism, even a large time delay may not necessarily lead to instability. We also show that, in case the time delay affects positive feedback (such as cooperation), the population dynamics can lead to self-organized formation of intermediate quasi-stationary states. Finally, we discuss ecological implications of our findings.








Similar content being viewed by others
Notes
Direct density dependence is often referred to as a first-order dynamics, whilst delayed density dependence is known as second-order (or higher) dynamics, see Turchin (1999)
References
Abbott O H, McNeilly A S, Lunn S F, Hulme M J, Burden F J (1981) Inhibition of ovarian function in subordinate marmoset monkeys (Callithrix jacchus). J Reprod Fertil 63 (2): 335–345
Adamson M W, Morozov A Y (2013) When can we trust our model predictions? Unearthing structural sensitivity in biological systems. Proc Roy Soc A: Math Phys Eng Sci 469 (2149)
Amarasekare P (1998a) Interactions between local dynamics and dispersal: Insights from single species models. Theor Popul Biol 53 (1): 44–59
Amarasekare P (1998b) Allee effects in metapopulation dynamics. Amer Nat 152 (2): 298–302
Asa C S, Valdespino C (1998) Canid reproductive biology: an integration of proximate mechanisms and ultimate causes. Amer Zool 38 (1): 251–259
Beddington J R, May R M (1975) Time delays are not necessarily destabilizing. Math Biosci 27: 109–117
Beretta E, Kuang Y (1998) Global analyses in some delayed ratio-dependent predator-prey systems. Nonlinear Anal Theory Methods Appl 32 (3): 381–408
Berryman A, Turchin P (2001) Identifying the density-dependent structure underlying ecological time series. Oikos 92: 265–270
Berryman A, Turchin P (1997) Detection of delayed density dependence: Comment. Ecology 78: 318–320
Berryman AA, Stenseth NC, Isaev AS (1987) Natural regulation of herbivorous forest insect populations. Oecologia 71: 174–184
Bjørnstad O N, Begon M, Stenseth N C, Falk W, Sait S M, Thompson D J (1998) Population dynamics of the Indian meal moth: demographic stochasticity and delayed regulatory mechanisms. J Anim Ecol 67: 110–126
Boukal D S, Berec L (2002) Single species models of the Allee effect: Extinction boundaries, sex ratios and mate encounters. J Theor Biol 218: 375–394
Briggs C J, Sait S M, Begon M, Thompson D J, Godfray H C J (2000) What causes generation cycles in populations of stored-product moths? J Anim Ecol 69 (2): 352–366
Buckland ST, Newman KB, Fernandez C, Thomas L, Harwood J (2007) Embedding population dynamics models in inference. Stat Sci 22 (1): 44–58
Cao Y, Gard T C (1995) Ultimate bounds and global asymptotic stability for differential delay equations. Rocky Mt J Math 25 (1): 119–131
Çelik C, Merdan H, Duman O, Akin Ö (2008) Allee effects on population dynamics with delay. Chaos, Solitons Fractals 37: 65–74
Chapman R N (1928) The quantitative analysis of environmental factors. Ecology 9: 111–122
Clark J, Bjørnstad O (2004) Population time series: process variability, observation errors, missing values, lags and hidden states. Ecology 85: 3140–3150
Clutton-Brock T H, Brotherton P N M, Smith R, McIlrath G M, Kansky R, Gaynor D, O’Riain M J, Skinner J D (1998) Infanticide and expulsion of females in a cooperative mammal. Proc Biol Sci 265 (1412): 2291–2295
Clutton-Brock T H, Hodge S J, Flower T P (2008) Group size and the suppression of subordinate reproduction in Kalahari meerkats. Anim Behav 76 (3): 689–700
Cooke K L, Grossman Z (1982) Discrete delay, distributed delay and stability switches. J Math Anal Appl 86: 592–627
Courchamp F, Berec L, Gascoigne J (2008) Allee effects in ecology and conservation. Oxford University Press
Courchamp F, Clutton-Brock T, Grenfell B (1999) Inverse density dependence and the Allee effect. Trends Ecol Evol 14 (10): 405–410
Cushing J M (1976) Predator-prey interactions with time delays. J Math Biol 3–4: 369–380
Cushing J M (1994) Oscillations in age-structured population models with an Allee effect. J Comput Appl Math 52: 71–80
De Roos A M, Persson L (2002) Size-dependent life-history traits promote catastrophic collapses of top predators. Proc National Acad Sci 99 (20): 12907–12912
De Roos A M, Persson L, Thieme H R (2003) Emergent Allee effects in top predators feeding on structured prey populations. Proc Royal Soc B 270: 611–618
Dennis B (1989) Allee effects: population growth, critical density and the chance of extinction. Nat Res Model 3: 481–538
Desharnais R A (1997) Population dynamics of Tribolium. In: Tuljapurkar S, Caswell H (eds) Structured population models in marine, terrestrial, and freshwater systems. Chapman & Hall, New York, pp 303–328
Elton C S (1924) Periodic fluctuations in the number of animals: their causes and effects. Br J Exper Biol 2: 119–163
Fagan W F, Lewis M A, Neurbert M G, van der Driessche P (2002) Invasion theory and biological control. Ecol Lett 5: 148-157
Fowler M S, Ruxton G D (2002) Population dynamic consequences of Allee effects. J Theor Biol 215: 39–46
Freedman H I, Gopalsamy K (1986) Global stability in time delayed single species dynamics. Bull Math Biol 48 (5): 485–492
Fussmann G F, Blasius B (2005) Community response to enrichment is highly sensitie to model structure. Biol Lett 1: 9–12
Ginzburg L R, Taneyhill D E (1994) Population cycles of forest Lepidoptera: a maternal effect hypothesis. J Anim Ecol 63: 79–92
Gonzales-Andujar J L, Fernandes Quintanilla C, Navarrete L (2006) Population cycles produced by delayed density dependence in an annual plant. Amer Natur 168: 318–322
Gopalsamy K, Ladas G (1990) On the oscillation and asymptotic behaviour of Ṅ(t) = N(t)[a + bN(t −T ) − cN 2 (t −T )]. Q Appl Math 3: 433–440
Graham I S, Lambin X (2002) The impact of weasel predation on cyclic field vole survival: the specialist predator hypothesis contradicted. J Anim Ecol 71 (6): 946–956
Griffin A S, Pemberton J M, Brotherton P N M, McIlrath G, Gaynor D, Kansky R, O’Riain J, Clutton-Brock T H (2003) A genetic analysisof breeding success in the cooperative meerkat (Suricatasuricatta). Behav Ecol 14: 472–480
Griffiths S W, Armstrong J D, Metcalfe N B (2003) The cost of aggregation: Juvenile salmon avoid sharing winter refuges with siblings. Behav Ecol 14 (5): 202–206
Hansen T F, Stenseth N C, Henttonen H, Tast J (1998) Interspecific and intraspecific competition as causes of direct and delayed density dependence in a fluctuating vole population. Proc National Acad Sci USA 96 (3): 986–991
Hastings A (1984) Delays in recruitment at different trophic levels: effects on stability. J Math Biol 21 (1): 35–44
Hutchinson G E (1948) Circular causal systems in ecology. Annals New York Acad Sci 50: 221–246
Koenig W D, Dickinson J L (2004) Ecology and evolution of cooperative breeding in birds. Cambridge University Press
Kot M (2001) Elements of mathematical ecology. Cambridge University Press, Cambridge
Kramer A M, Dennis B, Liebhold A M, Drake J M (2009) The evidence for Allee effects. Popul Ecol 51: 341–354
Kramer K L (2010) Cooperative breeding and its significance to the demographic success of humans. Annu Rev Anthropol 39: 417–436
Krebs C J (1996) Population cycles revisited. J Mammal 77 (1): 8–24
Krebs C J, Myers J (1974) Population cycles in small mammals. Adv Ecol Res 8: 267–399
Kuang Y (1993) Delay differential equations with applications in population dynamics. Academic Press, New York
Lewis M A, Kareiva P (1993) Allee dynamics and the spread of invading organisms. Theor Popul Biol 43: 141–158
Lewis M A, van den Driessche P (1993) Waves of extinction from sterile insect release. Math Biosci 116: 221–247
Levins R (1969) Some demographic and genetic consequences of environmental heterogeneity for biological control. Bull Entomol Soc Am 15: 237-240
Liebhold A M, Bascompte J (2003) The Allee effect, stochastic dynamics and the eradication of alien species. Ecol Lett 6: 133–140
Liebhold A M, Tobin P C (2010) Exploiting the Achilles heels of pest invasions: Allee effects, stratified dispersal and management of forest insect establishment and spread. N Z J Forest Sci 40: S25–S33
Liz E, Pinto M, Robledo G, Trofimchuk S, Tkachenko V (2003) Wright type delay differential equations with negative Schwarzian. Discret Contin Dyn Syst 9: 309–321
May R M (1973) Time delay versus stability in population models with two and three trophic levels. Ecology 54 (2): 315–325
Maynard Smith J (1974) Models in ecology. Cambridge University Press
Merdan H, Duman O, Akin Ö, Çelik C (2009) Allee effects on population dynamics in continuous (overlapping) case. Chaos, Solitons Fractals 39: 1994–2001
Merdan H, Gümüş Ö (2012) Stability analysis of a general discrete-time population model involving delay and Allee effects. Appl Math Comput 219: 1821–1832
Morozov A Y, Petrovskii S V, Li B-L (2006) Spatiotemporal complexity of the patchy invasion in a predatorprey system with the Allee effect. J Theor Biol 238: 18-35
Murray JD (1989) Mathematical biology I an introduction. Springer
Nakamura K, Hasan N, Abbas I H, Godfray C J, Bonsall M B (2004) Generation cycles in Indonesian lady beetle populations may occur as a result of cannibalism. Proc Biol Sci 271 (6): S501–S504
Nakaoka S, Wang W, Takeuchi Y (2009) Effect of parental care and aggregation on population dynamics. J Theor Biol 260: 161– 171
Park T (1932) Studies in population physiology: the relation of numbers to initial population growth in the flour beetle. Tribolium confusum Duval Ecol 13: 172–181
Petrovskii S V, Morozov A Y, Venturino E (2002) Allee effect makes possible patchy invasion in a predator-prey system. Ecol Lett 5: 345–352
Petrovskii S V, Malchow H, Hilker F M, Venturino E (2005a) Patterns of patchy spread in deterministic and stochastic models of biological invasion and biological control. Biol Invasions 7: 771–793
Petrovskii S V, Morozov A Y, Li B L (2005b) Regimes of biological invasion in a prey predator system with the Allee effect. Bull Math Biol 67: 637–661
Petrovskii S, Blackshaw R, Li B L (2008) Consequences of the Allee effect and intraspecific competition on population persistence under adverse environmental conditions. Bull Math Biol 70 (2): 412–437
Rossiter M C (1991) Environmentally-based maternal effects: a hidden force in insect population dynamics. Oecologia 87 (2): 288–294
Ruan S G (1995) The effect of delays on stability and persistence in plankton models. Nonlinear Anal Theory Methods Appl 24: 575–585
Scheuring I (1999) Allee effect increases the dynamical stability of populations. J Theor Biol 199: 407–414
Smith M J, White A, Lambin X, Sheratt J A, Begon M (2006) Delayed-density dependent season length alone can lead to rodent population cycles. Am Nat 167 (5): 695–704
Snowdon C T, Pickhard J J (1999) Family feuds: Severe aggression among cooperatively breeding cotton top tamarins. Int J Primatol 20 (5): 651–663
So J W H, Wu J, Zou W (2001) Structured population on two patches: Modeling dispersal and delay. J Math Biol 43: 37–51
Solomon N G, French J A (1997) Cooperative breeding in mammals. Cambridge University Press
Stephens P A, Sutherland W J, Freckleton R P (1999) What is the Allee effect? Oikos 87 (1): 185–190
Taylor C M, Hastings A (2005) Allee effects in biological invasions. Ecol Lett 8: 895–908
Terry A J (2011) Dynamics of a structured population on two patches. J Math Anal Appl 378: 1–15
Tobin P C, Robinet C, Johnson D M, Whitmire S L, O N Bjørnstad, Liebhold A M (2009) The role of Allee effects in gypsy moth(Lymantria dispar (L.)) invasions. Popul Ecol 51: 373–384
Turchin P (1999) Population regulation: a synthetic view. Oikos 84 (1): 153–159
Turchin P (2003) Complex population dynamics: a theoretical/empirical synthesis. Princeton University Press
Turchin P, Taylor A D, Reeve J D (1999) Dynamical role of predators in population cycles of a forest insect: an experimental test. Science 285 (5430): 1068–1071
Van Kooten T, De Roos A M, Persson L (2005) Bistability and an Allee effect as emergent consequences of stage-specific predation. J Theor Biol 237: 67–74
Vercken E, Kramer A M, Tobin P C, Drake J M (2011) Critical patch size generated by Allee effect in gypsy moth, Lymantria dispar (L.) Ecol Lett 14: 179–186
Walsh R K, Bradley C, Apperson C S, Gould F (2012) An experimental field study of delayed density dependence in natural populations of Aedes albopictus. PLoS ONE 7 (4): e35959. 10.1371/journal.pone.0035959
Wiley H R, Rabenold K N (1984) The evolution of cooperative breeding by delayed reciprocity and queuing for favourable social positions. Evolution 38 (3): 609–621
Williams D W, Liebhold A M (1995) Detection of delayed density dependence: effects of autocorrelation in an exogenous factor. Ecology 76: 1005–1008
Young A J, Carlson A A, Monfort S L, Russell A F, Bennett N C, Clutton-Brock T H (2006) Stress and the suppression of subordinate reproduction in cooperatively breeding meerkats. Proc Nat Acad Sci USA 103 (32): 12005–12010
Acknowledgments
S.P. gratefully acknowledges the support given by the University of Leicester in granting an academic study leave which was essential for the completion of this work.
Author information
Authors and Affiliations
Corresponding author
Appendices
Appendix 1: Linear stability analysis
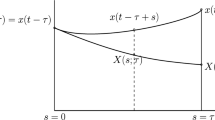
The general approach adopted in this and the following appendix is based on the standard linearization technique coupled with basic knowledge of ODE theory used to obtain a bifurcation value of the time delay parameter, τ. Since the method in question can be easily extended and applied to all models introduced in this paper, stability analysis will be shown for one model (Eq. 5) as an illustrative example, and results for other models listed. Stability of the upper positive equilibrium is considered as it is known that the intermediate equilibrium U ∗=β is always unstable.
Suppose x is a small perturbation from the steady state U ∗=K=1:
To determine equilibrium stability, we investigate the behavior of this small perturbation, whether it grows or decays, and for that purpose we linearize around the steady state, ignore higher order terms of Eq. 5, which gives:
Note that we treat the delayed variables, U(t−τ)=U τ and x(t−τ)=x τ , as separate variables throughout linearising. We look for solutions of form x=c e λt, where c is a constant and λ are the eigenvalues of the system which determine its stability. By substituting this solution form in Eq. 15, we obtain the transcendental characteristic equation:
for which analytical solutions are difficult to find. Nevertheless, from a stability viewpoint it is important to find whether there are any solutions with R e λ>0 which implies instability (since the perturbation grows exponentially with time). By setting λ=i ω, we assume R e λ=0 and obtain an expression for the critical value at which the multiplicative system passes through the Hopf bifurcation, below which stability prevails and above which instability occurs. Substituting into Eq. 16 and separating real and imaginary parts of the transcendental equation, we obtain the system:
Following some elementary calculations, we are able to find the critical value of τ:
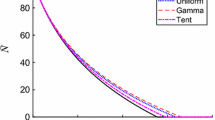
In all subsequent analysis, the characteristic of growth rate was taken as γ=1. In the below table, we summarize the stability conditions for all other models used.
For the delayed cooperation (Eq. 6), stability analysis confirmed that the equilibrium remains stable as:
thus the eigenvalue is always negative for all biologically viable values of β. Loss of stability does not occur for the model incorporating time delay in the maturation term (Eq. 10), as the stability condition in this case reads:
Since −1≤β≤0.5, \(\arccos \left (\frac {2}{1+\beta }\right )\) is not defined, there are no bifurcation values, τ c . As for the additive model incorporating time delay in direct competition (11), we were able to map all critical values, τ c :
with the exception of β=−1 as it is a singularity. Addition of another delayed term into direct competition results in Eq. (12) for which the stability condition reads:
The analytical bifurcation value, τ c , for the model with two delayed terms (13) the same as in model with delayed competition (5).
Appendix 2: Loss of monotonicity analysis
Following the linearization method previously described, we obtain expressions (and subsequently values) for the time delay parameter, τ, when the system loses monotonicity, i.e., when the solution still approaches the stable equilibrium U ∗=K, but in a nonmonotonous manner (referred to as damped oscillations). We introduce the conditions of monotonicity, solving for one model, and list results for all others, as above.
We consider Eq. 5 and its corresponding characteristic equation:
In general, whether the solution is monotone or not depends on the roots of this eigenvalue equation and loss of monotonicity is associated with the total loss of all relevant real eigenvalues. Obviously, when τ=0, the eigenvalue is negative for all biologically relevant values of β, with the exception for β=−1, in which case stability of system cannot be inferred. By increasing the time delay, the eigenvalue goes complex, at a value we will denote by τ ∗. At this critical value, the two curves intersect and the following condition holds:
On elimination of λ, using elementary calculus, one finds an explicit condition for τ:
and so loss of monotonicity is predicted for τ≥τ ∗. Note, this is the only case in which we may obtain an explicit expression, as with all other models implicit expressions are presented. In the below table, we summarize all critical values, τ ∗, for which solutions lose monotonicity for all subsequent models.
In Eq. 6, the solution remains monotonous as the eigenvalues are always negative for all applicable values of the Allee threshold (λ=β−1).
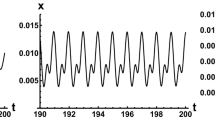
Equation 10, yielding the most interesting step-like results, clearly preserves monotonicity throughout all possible values of the time delay parameter, τ, as the monotonicity condition does not have any roots and reads:
For Eq. 11, the implicit expression for the critical time delay is:
A differing monotonicity condition is obtained for a fully delayed density dependent mortality (Eq. 12):
Our two delay model yielded the same condition as in Eq. 5, as was expected and confirmed in numerical simulations.
Rights and permissions
About this article
Cite this article
Jankovic, M., Petrovskii, S. Are time delays always destabilizing? Revisiting the role of time delays and the Allee effect. Theor Ecol 7, 335–349 (2014). https://doi.org/10.1007/s12080-014-0222-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12080-014-0222-z