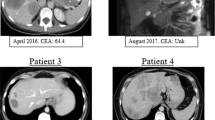
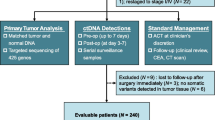
Abstract
Objective
To evaluate the predictive value of pre-hepatectomy dynamic circulating tumor DNA (ctDNA) on pathologic response to preoperative chemotherapy and recurrence after liver resection for colorectal liver metastases (CRLM).
Background
Pathologic response is a predictor of clinical outcomes for patients undergoing hepatectomy for CRLM. Postoperative ctDNA has been proven to be sensitive for recurrence detection. However, few studies investigate the impact of pre-hepatectomy ctDNA on pathologic response and recurrence.
Methods
Patients with potential resectable CRLM underwent preoperative chemotherapy and hepatectomy between 2018 and 2021 was considered for inclusion. Plasma ctDNA was collected before and after preoperative chemotherapy. Pathologic response was analyzed for all patients after liver resection. Recurrence free survival was compared between patients with different ctDNA status and different pathologic response. The relation between ctDNA and pathologic response was also analyzed.
Results
A total of 114 patients were included. ctDNA was detectable in 108 of 114 patients (94.7%) before chemotherapy, in 56 of 114 patients (49.1%) after chemotherapy. Patients with ctDNA positive at baseline and negative after chemotherapy had significantly longer RFS (median RFS 17 vs 7 months, p = 0.001) and HRFS (median HRFS unreached vs 8 months, p < 0.001) than those with ctDNA persistently positive after chemotherapy. Two patients (1.6%) had a pathologic complete response and 56 patients (45.2%) had a pathologic major response. Post-chemotherapy ctDNA− was associated with improved major pathologic response (53.4% vs 32.1%, p = 0.011). In the multivariable analysis, ctDNA− after chemotherapy (HR 0.51, 95% CI 0.28–0.93), major pathologic response (HR 0.34, 95% CI 0.19–0.62) and surgery combined with radiofrequency ablation (HR 2.62, 95% CI 1.38–5.00) were independently associated with RFS (all p < 0.05).
Conclusions
Pre-hepatectomy dynamic monitoring of ctDNA could predict pathologic response to preoperative chemotherapy and post-hepatectomy recurrence in CRLM patients. Negative ctDNA after preoperative chemotherapy was associated with better tumor regression grade and recurrence-free survival, which might be used to guide pre-hepatectomy chemotherapy and predict prognosis.





Similar content being viewed by others
Data availability
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.
References
Bray F, Ferlay J, Soerjomataram I, et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394–424
Chen W, Zheng R, Baade PD, et al. Cancer statistics in China, 2015. CA Cancer J Clin. 2016;66:115–132
Engstrand J, Nilsson H, Stromberg C, et al. Colorectal cancer liver metastases—a population-based study on incidence, management and survival. BMC Cancer. 2018;18:78
Reboux N, Jooste V, Goungounga J, et al. Incidence and survival in synchronous and metachronous liver metastases from colorectal cancer. JAMA Netw Open. 2022;5: e2236666
Osterlund P, Salminen T, Soveri LM, et al. Repeated centralized multidisciplinary team assessment of resectability, clinical behavior, and outcomes in 1086 Finnish metastatic colorectal cancer patients (RAXO): a nationwide prospective intervention study. Lancet Reg Health Eur. 2021;3: 100049
Liu W, Liu JM, Wang K, et al. Recurrent colorectal liver metastasis patients could benefit from repeat hepatic resection. BMC Surg. 2021;21:327
Berardi G, Chou J, Gonen M, et al. A model to predict treatment failure in patients undergoing upfront surgery for resectable colorectal liver metastases. Ann Surg Oncol. 2023;30:2820–2827
Rhaiem R, Rached L, Tashkandi A, et al. Implications of RAS mutations on oncological outcomes of surgical resection and thermal ablation techniques in the treatment of colorectal liver metastases. Cancers (Basel). 2022;14:816
Cervantes A, Adam R, Rosello S, et al. Metastatic colorectal cancer: ESMO Clinical Practice Guideline for diagnosis, treatment and follow-up. Ann Oncol. 2023;34:10–32
Rubbia-Brandt L, Giostra E, Brezault C, et al. Importance of histological tumor response assessment in predicting the outcome in patients with colorectal liver metastases treated with neo-adjuvant chemotherapy followed by liver surgery. Ann Oncol. 2007;18:299–304
Blazer DG 3rd, Kishi Y, Maru DM, et al. Pathologic response to preoperative chemotherapy: a new outcome end point after resection of hepatic colorectal metastases. J Clin Oncol. 2008;26:5344–5351
Takahashi T, Ishida K, Emi Y, et al. Pathological evaluation of resected colorectal liver metastases: mFOLFOX6 plus bevacizumab versus mFOLFOX6 plus cetuximab in the phase II ATOM trial. Cancers (Basel). 2022;14:4392
Mason MC, Krasnodebski M, Hester CA, et al. Outcomes of mixed pathologic response in patients with multiple colorectal liver metastases treated with neoadjuvant chemotherapy and liver resection. Ann Surg Oncol. 2022;29:5156–5164
Xu Y, He J, Li W, et al. The pathologic complete response ratio of liver metastases represents a valuable prognostic indicator. Pathol Oncol Res. 2022;28: 1610663
Xu D, Wang YY, Yan XL, et al. Development of a model to predict pathologic response to chemotherapy in patients with colorectal liver metastases. J Gastrointest Oncol. 2021;12:1498–1508
Zhu HB, Xu D, Ye M, et al. Deep learning-assisted magnetic resonance imaging prediction of tumor response to chemotherapy in patients with colorectal liver metastases. Int J Cancer. 2021;148:1717–1730
Adams AM, Vreeland TJ, Newhook TE. Circulating tumor DNA: towards more individualized treatment for patients with resectable colorectal cancer. J Gastrointest Cancer. 2023;54:1071–1081
Marmorino F, Prisciandaro M, Giordano M, et al. Circulating tumor DNA as a marker of minimal residual disease after radical resection of colorectal liver metastases. JCO Precis Oncol. 2022;6: e2200244
Newhook TE, Overman MJ, Chun YS, et al. Prospective study of perioperative circulating tumor DNA dynamics in patients undergoing hepatectomy for colorectal liver metastases. Ann Surg. 2023;277:813–820
Wang DS, Yang H, Liu XY, et al. Dynamic monitoring of circulating tumor DNA to predict prognosis and efficacy of adjuvant chemotherapy after resection of colorectal liver metastases. Theranostics. 2021;11:7018–7028
Ogaard N, Reinert T, Henriksen TV, et al. Tumour-agnostic circulating tumour DNA analysis for improved recurrence surveillance after resection of colorectal liver metastases: a prospective cohort study. Eur J Cancer. 2022;163:163–176
Evrard S, Torzilli G, Caballero C, et al. Parenchymal sparing surgery brings treatment of colorectal liver metastases into the precision medicine era. Eur J Cancer. 2018;104:195–200
Liu M, Wang Y, Wang K, et al. Combined ablation and resection (CARe) for resectable colorectal cancer liver metastases—a propensity score matching study. Eur J Surg Oncol. 2023;49: 106931
Liu W, Jin KM, Zhang MH, et al. Recurrence prediction by circulating tumor DNA in the patient with colorectal liver metastases after hepatectomy: a prospective biomarker study. Ann Surg Oncol. 2023;30:4916–4926
Reinert T, Petersen LMS, Henriksen TV, et al. Circulating tumor DNA for prognosis assessment and postoperative management after curative-intent resection of colorectal liver metastases. Int J Cancer. 2022;150:1537–1548
Tie J, Wang Y, Cohen J, et al. Circulating tumor DNA dynamics and recurrence risk in patients undergoing curative intent resection of colorectal cancer liver metastases: a prospective cohort study. PLoS Med. 2021;18: e1003620
Kobayashi S, Nakamura Y, Taniguchi H, et al. Impact of preoperative circulating tumor DNA status on survival outcomes after hepatectomy for resectable colorectal liver metastases. Ann Surg Oncol. 2021;28:4744–4755
Bidard FC, Kiavue N, Ychou M, et al. Circulating tumor cells and circulating tumor DNA detection in potentially resectable metastatic colorectal cancer: a prospective ancillary study to the Unicancer Prodige-14 trial. Cells. 2019;8:516
Vega EA, Salehi O, Nicolaescu D, et al. Failure to cure patients with colorectal liver metastases: the impact of the liver surgeon. Ann Surg Oncol. 2021;28:7698–7706
Xu D, Yan XL, Liu JM, et al. The characteristics and long-term survival of patients with colorectal liver metastases with pathological complete response after chemotherapy. J Cancer. 2020;11:6256–6263
Huang Y, Jia W, Wang L, et al. Clearance of circulating tumor DNA in a high-risk stage-IV rectal carcinoma patient with synchronous liver metastases after conversion surgery is correlated with pathologic complete response. Ther Adv Gastrointest Endosc. 2021;14: 26317745211020279
Funding
This research was supported by the Clinical Research Fund For Distinguished Young Scholars of Beijing Cancer Hospital (No. LGH2022005) and grants from the National Nature Science Foundation of China (No. 31971192).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
There are no conflicts of interest for all the authors who participate in this study, including Ming Liu, Quan Bao, Tingting Zhao, Longfei Huang, Danhua Zhang, Yanyan Wang, Xiaoluan Yan, Hongwei Wang, Kemin Jin, Wei Liu, Kun Wang and Baocai Xing.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
12072_2023_10628_MOESM2_ESM.tiff
Fig. S2 Plots of HRFS stratified by ctDNA fraction. A) HRFS stratified by ctDNA dynamics peri-chemotherapy, ctDNA +/-, ctDNA -/- and ctDNA +/+. B) HRFS stratified by baseline ctDNA status. C) HRFS stratified by the post-chemotherapy ctDNA status. (TIFF 33973 KB)
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Liu, M., Bao, Q., Zhao, T. et al. Pre-hepatectomy dynamic circulating tumor DNA to predict pathologic response to preoperative chemotherapy and post-hepatectomy recurrence in patients with colorectal liver metastases. Hepatol Int (2024). https://doi.org/10.1007/s12072-023-10628-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12072-023-10628-4