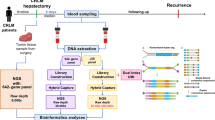
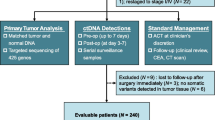
Abstract
Background
Minimal residual disease (MRD) is proposed to be responsible for tumor recurrence. The role of circulating tumor DNA (ctDNA) to detect MRD, monitor recurrence, and predict prognosis in liver cancer patients undergoing liver transplantation (LT) remains unrevealed.
Methods
Serial blood samples were collected to profile ctDNA mutational changes. Baseline ctDNA mutational profiles were compared with those of matched tumor tissues. Correlations between ctDNA status and recurrence rate (RR) and recurrence-free survival (RFS) were analyzed, respectively. Dynamic change of ctDNA was monitored to predict tumor recurrence.
Results
Baseline mutational profiles of ctDNA were highly concordant with those of tumor tissues (median, 89.85%; range 46.2–100%) in the 74 patients. Before LT, positive ctDNA status was associated with higher RR (31.7% vs 11.5%; p = 0.001) and shorter RFS than negative ctDNA status (17.8 vs 19.4 months; p = 0.019). After LT, the percentage of ctDNA positivity decreased (17.6% vs 47.0%; p < 0.001) and patients with positive ctDNA status had higher RR (46.2% vs 21.3%; p < 0.001) and shorter RFS (17.2 vs 19.2 months; p = 0.010). Serial ctDNA profiling demonstrated patients with decreased or constant negative ctDNA status had lower RR (33.3% vs 50.0%; p = 0.015) and favorable RFS (18.2 vs 15.0 months, p = 0.003) than those with increased or constant positive ctDNA status. Serial ctDNA profiling predicted recurrence months ahead of imaging evidence and serum tumor biomarkers.
Conclusions
ctDNA could effectively detect MRD and predict tumor recurrence in liver cancer patients undergone LT.





Similar content being viewed by others
Data availability
The raw data generated in this study are not publicly available since the sequencing used in this study was a commercial service paid by the patients. However, the derived data supporting the findings of this study are available from the corresponding author upon reasonable request.
References
Sapisochin G, Bruix J. Liver transplantation for hepatocellular carcinoma: outcomes and novel surgical approaches. Nat Rev Gastroenterol Hepatol 2017;14:203–217
Ju MR, Yopp AC. Evolving thresholds for liver transplantation in hepatocellular carcinoma: a Western experience. Ann Gastroenterol Surg 2020;4:208–215
Beal EW, Cloyd JM, Pawlik TM. Surgical treatment of intrahepatic cholangiocarcinoma: current and emerging principles. J Clin Med 2020;10:104
Zhang Y, Shi ZL, Yang X, et al. Targeting of circulating hepatocellular carcinoma cells to prevent postoperative recurrence and metastasis. World J Gastroenterol 2014;20:142–147
Janni W, Rack B, Kasprowicz N, et al. DTCs in breast cancer: clinical research and practice. Recent results in cancer research. Fortschritte der Krebsforschung Progres dans les recherches sur le Cancer 2012;195:173–178
Wan JCM, Mughal TI, Razavi P, et al. Liquid biopsies for residual disease and recurrence. Med (New York, NY) 2021;2:1292–1313
Honoré N, Galot R, van Marcke C, et al. Liquid biopsy to detect minimal residual disease: methodology and impact. Cancers 2021;13:5364
Siravegna G, Mussolin B, Venesio T, et al. How liquid biopsies can change clinical practice in oncology. Ann Oncol 2019;30:1580–1590
von Felden J, Garcia-Lezana T, Schulze K, et al. Liquid biopsy in the clinical management of hepatocellular carcinoma. Gut 2020;69:2025–2034
Chen Z, Lin X, Chen C, et al. Analysis of preoperative circulating tumor cells for recurrence in patients with hepatocellular carcinoma after liver transplantation. Ann Transl Med 2020;8:1067
Wang PX, Xu Y, Sun YF, et al. Detection of circulating tumour cells enables early recurrence prediction in hepatocellular carcinoma patients undergoing liver transplantation. Liver Int 2021;41:562–573
Huang A, Guo DZ, Wang YP, et al. Plasma MicroRNA panel predicts early tumor recurrence in patients with hepatocellular carcinoma after liver transplantation. J Cancer 2021;12:7190–7200
Zhou J, Sun H, Wang Z, et al. Guidelines for the diagnosis and treatment of hepatocellular carcinoma (2019 edition). Liver Cancer 2020;9:682–720
Li J, Jiang W, Wei J, et al. Patient specific circulating tumor DNA fingerprints to monitor treatment response across multiple tumors. J Transl Med 2020;18:293
Odegaard JI, Vincent JJ, Mortimer S, et al. Validation of a plasma-based comprehensive cancer genotyping assay utilizing orthogonal tissue- and plasma-based methodologies. Clin Cancer Res 2018;24:3539–3549
Koboldt DC, Zhang Q, Larson DE, et al. VarScan 2: somatic mutation and copy number alteration discovery in cancer by exome sequencing. Genome Res 2012;22:568–576
Yang JD, Hainaut P, Gores GJ, et al. A global view of hepatocellular carcinoma: trends, risk, prevention and management. Nat Rev Gastroenterol Hepatol 2019;16:589–604
Halazun KJ, Najjar M, Abdelmessih RM, et al. Recurrence after liver transplantation for hepatocellular carcinoma: a new MORAL to the story. Ann Surg 2017;265:557–564
von Felden J, Villanueva A. Role of molecular biomarkers in liver transplantation for hepatocellular carcinoma. Liver Transpl 2020;26:823–831
Akbulut S, Koc C. Do we need to be limited by matching milan criteria for survival in living donor liver transplantation? J Gastrointest Cancer 2020;51:1107–1113
Sarici B, Isik B, Yilmaz S. Management of recurrent HCC after liver transplantation. J Gastrointest Cancer 2020;51:1197–1199
Peng Y, Mei W, Ma K, et al. Circulating tumor DNA and minimal residual disease (MRD) in solid tumors: current horizons and future perspectives. Front Oncol 2021;11: 763790
Amado V, González-Rubio S, Zamora J, et al. Clearance of circulating tumor cells in patients with hepatocellular carcinoma undergoing surgical resection or liver transplantation. Cancers 2021;13:2476
Huang A, Zhang X, Zhou SL, et al. Detecting circulating tumor dna in hepatocellular carcinoma patients using droplet digital PCR is feasible and reflects intratumoral heterogeneity. J Cancer 2016;7:1907–1914
Wang J, Huang A, Wang YP, et al. Circulating tumor DNA correlates with microvascular invasion and predicts tumor recurrence of hepatocellular carcinoma. Ann Transl Med 2020;8:237
Huang A, Zhao X, Yang XR, et al. Circumventing intratumoral heterogeneity to identify potential therapeutic targets in hepatocellular carcinoma. J Hepatol 2017;67:293–301
Ao H, Xin Z, Jian Z. Liquid biopsy to identify biomarkers for immunotherapy in hepatocellular carcinoma. Biomark Res 2021;9:91
Deng Y, Sun L, Liang H, et al. Measurement of circulating tumor cells to track hepatocellular carcinoma progression after liver transplantation-case report. Front Oncol 2021;11: 760765
Funding
This study was jointly supported by Shanghai Municipal Health Commission (20224Y0285, R2022-010), National Natural Science Foundation of China (No.82150004), Natural Science Foundation of Shanghai (No.20ZR1473100), and Shanghai Municipal Key Clinical Specialty.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study’s conception and design. Material preparation, data collection and analysis were performed by AH, D-ZG, XZ, YS and S-YZ. The first draft of the manuscript was written by AH and XZ and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
Ao Huang, De-Zhen Guo, Xuan Zhang, Ying Sun, Shi-Yu Zhang, Xin Zhang, Xiu-Tao Fu, Yu-Peng Wang, Guo-Huan Yang, Qi-Man Sun, Yi-Feng He, Kang Song, Xiao-Wu Huang, Xin-Rong Yang, Wei-Ren Liu, Zhen-Bin Ding, Ying-Hong Shi and Jia Fan and Jian Zhou declares that they have no conflict of interest. The authors have no relevant financial or non-financial interests to disclose.
Ethical approval
All procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and with the Helsinki Declaration of 1975, as revised in 2008 (5). Informed consent was obtained from all patients for being included in the study.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Huang, A., Guo, DZ., Zhang, X. et al. Serial circulating tumor DNA profiling predicts tumor recurrence after liver transplantation for liver cancer. Hepatol Int 18, 254–264 (2024). https://doi.org/10.1007/s12072-023-10594-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12072-023-10594-x