Abstract
Purpose Leukoplakia is a macroscopic morphological term for thick white or grey mucosal patches that can represent various histologic diagnostic entities ranging from hyperplasia to malignancy. Aim was the study morphology of the superficial mucosa and microvascular network of the vocal cords in patients with suspected glottic squamous cell carcinoma (SCC) using contact endoscopy (CE). Material and Methods Seventy-nine patients (21 female, 58 male), with a mean age of 57.5 years ± 7.12 (range, 32–73 years), were prospectively enrolled and evaluated. Of these patients, 58 had leukoplakia (Group A/41 males and 17 females, with a mean age of 53.7 years ± 6.65), and 21 (Group B/ 17males and 4 females/ with a mean age of 60.5 years ± 6.04) had malignant lesions (pT1, n = 6; p T2, n = 8; pT3, n = 8; Group B), as proven by the results of the histological examination. Further, 79 non-smokers (control group—group C) were studied. CE imaging findings were classified into five types (I to V) based on the features of the mucosal intra-epithelial capillary loops. CE findings were correlated to the histologic findings. A separate analysis involving smoking status was done. Results The CE-based intraepithelial papillary capillary loop classification score was strongly correlated with the histological findings. Age was strongly associated with both malignancy and bilateral involvement. Smoking habits didn’t significantly differ between patients with unilateral and bilateral SCC. Conclusions CE imaging of the vocal cord mucosal capillaries may be useful for the early detection of glottic SCC and pre-cancerous lesions.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Leukoplakia is a macroscopic morphological term for thick white or grey mucosal patches that can represent various histologic diagnostic entities ranging from hyperplasia to malignancy [1,2,3,4]. Endoscopic (white light, WL) examination combined with stroboscopy is currently the standard core approach for detecting and assessing vocal cord leukoplakia (VFL) or other vocal cord lesions [5,6,7]. Contact endoscopy (CE) was originally described by Hamou in 1979 as a technique for the visualization of cervical and uterine epithelial cells for screening and diagnosis of cervical and uterine pathology [3]. Andrea et al. used CE to examine the vocal cords and nasal mucosa in the 1990s [8, 9]. Thereafter CE use has been reported in the diagnosis of mucosal lesions of the oral cavity [6], oropharynx, hypopharynx [7], nose [8], nasopharynx [9], recurrence of ear cholesteatoma [10], per operative identification of parathyroid glands [11], comparison of fungiform papillae during aging [11], in diabetes mellitus [12], in Bell’s palsy & herpes zoster [13], after the transaction of the chorda tympani facial nerve branch [12], in smokers and after smoking cessation [14].
The goal of this study was to evaluate distinct vascular patterns in patients with VFL and glottic squamous cell carcinoma (SCC) using contact endoscopy and investigate whether there are any correlations between the CE findings and the standard histologic findings in the mucosa of the vocal cord in these patients.
Materials and Methods
Clinical Epidemiologic Data
Seventy-nine patients (21 female, 58 male), with a mean age of 57.5 years ± 7.12 (range, 32–73 years), were prospectively enrolled and evaluated.
Of these patients, 58 had leukoplakia (Group A), and 21 had malignant lesions (pT1, n = 6; p T2, n = 8; pT3, n = 8; Group B), as proven by the results of the histological examination. Regarding the patients with malignancies, 8 had bilateral lesions, and thus 29 SCC lesions were studied and detected with CE. Between patients with leukoplakia and no malignancy, there was a total of 77 lesions. Therefore, a total of 106 lesions (29 malignancies/squamous cell carcinoma and 77 non-SCC) were found. Concerning the leukoplakia-patients, by 29 of them a low-grade dysplacia has been observed, and the rest of them suffered from a high-grade one. By the tumor-suffering patients, all of them had a high grade SCC.
In Table 2, demographic features, such as smoking status and age, of patients with and without SCC are depicted and compared.
For this study, we have additionally examined 79 non-smokers (control group–group C), who received total anesthesia for surgeries for non-otolaryngological diseases, such as hernias or other abdominal surgeries.
The study protocol was reviewed and approved by the Institutional Review Board ( Papanikolaou General Hospital, Thessaloniki, Nr. 72–2,204,202). All participants provided informed consent for participation in the study after being instructed about the aim and the procedures of the study. The procedures were in accordance with local data protection guidelines and legislation and according to the principles of the Helsinki Declaration for studies in humans.
Contact Endoscopy
For contact endoscopy the Andrea-Dias Contact Micro Laryngoscope (with HOPKINS Straight Forward Telescope 0° and 30°, with diameter 5.5 mm, length 23 cm, magnification 60 × and 150 ×); a 3-chip camera (Tricam SL II); a Xenon 175 Watt light source and a video recording system have been used, all manufactured by Karl Storz (Tuttlingen, Germany).
Vascular patterns were studied before staining because the staining dye causes a loss of transparency of the vocal cord mucosa making the blood vessels invisible. Epithelial cellular architecture was studied after staining the mucosal surface with 1% methylene blue which imparts a dark blue color to the nucleus and a light blue color to the cytoplasm. The excess stain has been removed by washing the area with a copious amount of saline using suction and irrigation. Methylene blue is non-toxic, and the staining is reversible [8, 9, 11
Endoscopically guided biopsy of laryngeal lesions was also performed under general anesthesia; tissue specimens were fixed in 10% formalin for histological analysis [10, 11]. The recorded CE findings were examined by two experienced professionals (PP and VST), who evaluated the pictures separately before discussing the results together. Both of them were blinded to the histological results at the time of assessment of the CE findings. The interrater reliability was calculated with the use of the Kappa test and was equal to 0.89 (Cohen’s kappa statistic).
Morphological Types of the Surface of the Vocal Cords
The morphological types of vocal cord leukoplakia assessed by preoperative rigid laryngoscopy were categorized as flat and smooth, elevated and smooth, and rough type [11, 12].
These definitions were based on the following morphological pattern categories, as depicted in Table 1.
Patterns and Changes
The Ni categorization was used for our research.16 Intraepithelial capillary loop alterations seen on CE can be categorized into five categories (I to V) according to this classification. Intraepithelial papillary capillary loops are nearly inconspicuous in type I, while oblique and arborescent capillaries of small diameter are discernible. The intraepithelial papillary capillary loops are nearly invisible in type II, while the diameter of the apparent oblique and arborescent capillaries is increased. The mucosa is white in type III, and the intraepithelial papillary capillary loops are invisible; if the white patch is thin, the oblique and arborescent vessels can be seen indistinctly, but if the white patch is thick, the vessels are obscured. The mucosal intraepithelial papillary capillary loops appear as scattered, small, dark brown spots in type IV, with a relatively regular arrangement and low density; the capillary terminals are bifurcated or slightly dilated, and the intraepithelial papillary capillary loops appear as scattered, small, dark brown spots; the oblique and arborescent vessels are usually not visible [13].
Type V changes are subdivided into types Va, Vb, and Vc according to the shape, regularity, and distribution of vessels. In type Va, intraepithelial papillary capillary loops are significantly dilated and of relatively high density, and appear to be solid or to have hollow, brownish, speckled features and various shapes [13]. In type Vb, the intraepithelial papillary capillary loop itself is destroyed, with its remnants presenting in a snake-, earthworm-, tadpole- or branch-like shape, and the microvessels are dilated, elongated, and ‘woven’ in appearance. In type Vc, the lesion surface is covered with necrotic tissue, and the intraepithelial papillary capillary loops present as brownish speckles or tortuous shapes of uneven density which are irregularly scattered on the tumor surface [13].
According to the shape, regularity, and distribution of vessels, type V changes are split into types Va, Vb, and Vc. Intraepithelial papillary capillary loops in type Va are highly dilated and of relatively high density, appearing solid or hollow, brownish, speckled, and of varied shapes.16 The intraepithelial papillary capillary loop is disrupted in type Vb, with remains resembling a snake, earthworm, tadpole, or branch, and microvessels that are dilated, elongated, and 'woven' in appearance. The lesion surface is coated with necrotic tissue in type Vc, and the intraepithelial papillary capillary loops appear as brownish speckles or sinuous shapes of uneven density spread irregularly on the tumor surface [12,13,14]. The various type categories are depicted in Figs. 1, 2, and 3.
The images depict type I, II, and III patterns of vascularization respectively. In type I, the intraepithelial papillary capillary loops are almost invisible; oblique and arborescent small-diameter vessels can be seen. In type II, the intraepithelial papillary capillary loops are also almost invisible, but the diameter of the observed oblique and arborescent vessels is enlarged. In type III, the mucosa is whitish and the intraepithelial papillary capillary loops cannot be seen; if the whitish patch is thin, the oblique and arborescent vessels may be seen indistinctly, but if the whitish patch is thick the vessels will be obscured.
The images depict type I, II, and III patterns of vascularization respectively. In type I, the intraepithelial papillary capillary loops are almost invisible; oblique and arborescent small-diameter vessels can be seen. In type II, the intraepithelial papillary capillary loops are also almost invisible, but the diameter of the observed oblique and arborescent vessels is enlarged. In type III, the mucosa is whitish and the intraepithelial papillary capillary loops cannot be seen; if the whitish patch is thin, the oblique and arborescent vessels may be seen indistinctly, but if the whitish patch is thick the vessels will be obscured.
The images depict type I, II, and III patterns of vascularization respectively. In type I, the intraepithelial papillary capillary loops are almost invisible; oblique and arborescent small-diameter vessels can be seen. In type II, the intraepithelial papillary capillary loops are also almost invisible, but the diameter of the observed oblique and arborescent vessels is enlarged. In type III, the mucosa is whitish and the intraepithelial papillary capillary loops cannot be seen; if the whitish patch is thin, the oblique and arborescent vessels may be seen indistinctly, but if the whitish patch is thick the vessels will be obscured.
Histologic Examination
All the tissues were processed for pathological testing on a standard basis. The same pathologist, who was blinded to the CE findings, evaluated and graded histologically graded formalin-fixed and paraffin-embedded slides independently. Squamous cell hyperplasia with non-dysplasia, mild dysplasia, moderate dysplasia, severe dysplasia, carcinoma in situ, and squamous cell carcinoma were all assessed histologically according to the World Health Organization's (WHO) 2017 guidelines [15]. The WHO 2017 classification is a two-tier system. Laryngeal precursor lesions are classified as low-grade dysplasia (previous categories squamous hyperplasia, mild dysplasia), and high-grade dysplasia (previous categories of moderate and severe dysplasia, carcinoma in situ). Carcinoma in situ is distinguished from high-grade dysplasia, showing features of conventional carcinoma [15].
Statistical Analysis
Parameters were evaluated using jamovi software (Version 1.6, Sydney, Australia from www.jamovi.org). A p-value less than 0.05 was considered statistically significant for all analyses. Independent samples t-test, Mann–Whitney U test, and Chi-square test were used for basic characteristics’ comparisons between male and female patients’ features (age, years of smoking, number of cigarettes/day) as well as for comparisons between patients with unilateral or bilateral lesions and patients with or without histologically confirmed malignancies. The association between the appearance of the surface of the vocal cords and the vascular patterns was examined in both groups of leukoplakia and glottic cancer, as well as in healthy vocal cords using the Chi-square test or Fisher’s exact test (if expected counts were less than 5). The association between the healthy cord’s vascularization pattern and the presence or absence of malignancy in the contralateral cord was investigated using the Chi-square test or Fisher’s exact test.
Results
Age was strongly associated with both malignancy and bilateral involvement, since in our studied cohort SCC lesions were much more prevalent in older rather than in younger participants (t = −4.11, p < 0.001). Participants with bilateral lesions were significantly older than those with unilateral disease (t = −2.06, p = 0.043). The grade of dysplasia by the patients (low or high) did not have any effect to the results.
The number of cigarettes smoked daily was significantly higher in the malignancies group compared to patients with no malignancy (U = 262, p < 0.001).
Regarding basic characteristics, there was no difference in age, number of cords affected, cigarettes smoked per year, and years smoking between male and female patients.
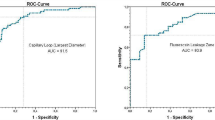
A Fisher exact test was performed to examine whether the results of intraepithelial papillary capillary loop classification using CE imaging and the morphological types of the lesions could be associated. There appears to be statistical significance, p < 0.001, suggesting that in general, there is an association between the macroscopic changes observed on the surface of the vocal folds and the method of CE (Table 2). Thus, “flat and smooth” or “elevated and smooth” vocal cords are more likely to be classified as type I, II, or III as regards vascular categorization, whereas the “rough type” correspond to vascular patterns IV and V. Furthermore, as it can be seen in Fig. 1, both methods were able to detect histologically confirmed malignancies.
A Fisher’s exact test was used indicating an agreement between the two methods regarding the categorization of vocal cord lesions (p < 0.001). fs = flat and smooth type; es = elevated and smooth type; r = rough type.
Lastly, we also wanted to test if there was a specific pattern between the vascularization type of the healthy vocal cord and the presence of malignancy in the contralateral cord of these patients. We used a Fisher’s exact test, which showed statistical significance (p < 0.001), suggesting that, as seen in Table 3, healthy cords with early stages of vascular classification (mostly type I) were more likely to belong to patients who have leukoplakia or non-malignant contralateral vocal cords. On the other hand, healthy cords of type III or IV were more likely to be associated with contralateral malignancies (Table 4).
Discussion
We provide evidence that vocal cord squamous cell carcinoma is associated with a single abnormal vascular pattern on the epithelial surface of the vocal cords, whereas leukoplakia may be associated with either normal or various abnormal vascular patterns. In addition, in patients with leukoplakia, the cumulative time spent smoking (in years) had a detrimental impact on the surface and vascularization of the vocal cords.
Capillaries in the superficial lamina propria, smaller arteries, and veins, as well as arterioles and venules in the deeper layers, characterize the vascular microanatomy of human vocal cords. Arterioles and venules have direct vascular anastomoses [16].
Under rigid laryngeal endoscopy, vocal cord leukoplakia presents as a white or grayish confined patch, distributed granule, or verrucous structure. It may have one or more localizations [10]. Leukoplakia is a chameleon-like epithelial transformation that can range from benign thickening to malignant tumors. As a result, the name "leukoplakia" is insufficient to characterize the lesion's histological identity [15,16,17,18].
There are previous reports on a tissue-specific classification of vascular changes associated with laryngeal leukoplakia. According to former reports, age, non-homogenous lesion texture, and the existence of hyperemia are independent predictors of malignancy [18,19,20]. These reports support the findings of the present study to a certain extent because they also provide evidence that age and lesion texture may predict prognosis. However, the impact of age on leukoplakia lesions has not been extensively explored. Our study provides preliminary evidence that age may be related to the development of this disease. Moreover, a further novelty of the present study is that a very detailed study on the lesion texture has been conducted. Leukoplakia lesions have traditionally been divided into two categories from their appearances which were individually homogenous and heterogeneous in many reports [21].
Although new endoscopic tools, such as narrow-band imaging, optical coherence tomography, and contact endoscopy have been developed to improve the diagnosis of vocal cord leukoplakia, WL laryngoscopy is the usual standard of care in clinical practice [22,23,24,25]. The ability of rigid or flexible laryngoscopy to visualize and characterize lesions of vocal cords continues to improve.
Many researchers have reported high efficacy of CE inthe diagnosis of mucosal lesions not only of the larynx but in other sites of head and neck mucosal surfaces as well [10,11,12,13, 26]. These results have been obtained by taking the histopathological examination as the gold standard. The technique of CE has definite advantages and limitations. Contact endoscopy enables visualization of tumor margins, dysplasia, and normal epithelium, thus offering the possibility of more precise complete removal of laryngeal lesions in a single sitting. Along with in vivo studies, contact endoscopy can also be used to analyze the excised segment of the lesion and hence ensure whether the lesion has been completely resected. The grade of dysplasia is indicated by the impaired nucleus/cytoplasm ratio, nuclear hyperchromasia, and variation in the number and appearance of the nucleoli [25, 27].
Of course, there are limitations in the use of CE, which should be also considered in the interpretation and validation of the results of the present study. Two inherent limitations are the inability to detect very early dysplasia and the inability to differentiate carcinoma in situ from invasive carcinoma.
Conclusion
Vascular changes may play an important role as one of the most prominent features in the endoscopic work-up of vocal cord lesions. Further validation of our preliminary findings, especially in combination with further standardized morphologic endoscopy findings (together with macroscopic appearance, mucosal vibration, vocal cord stiffness, and others) should be undertaken to increase the reliability of pre- and intraoperative diagnosis of leukoplakia and malignant glottic lesions.
Data availability
The data presented in this study are available at reasonable request from the corresponding author. The data are not publicly available due to ethical/privacy restrictions.
Code availability
Not applicable.
References
Gale N, Micjhaels L, Luzar B et al (2009) Current review on squamous intraepithelial lesions of the larynx. Histopathology 54:639–656
Ahn A, Wang L, Slaughter JC et al (2016) Serial full-thickness excision of dysplastic ocal cord leukoplakia: diagnostic or therapeutic? Laryngoscope 126:923–927
Li C, Zhang N, Wang S, Cheng L et al (2018) A new classification of vocal cord leukoplakia by morphological appearance guiding the treatment. Acta Otolaryngol 138:584–589
Chen M, Li C, Yang Y, Cheng L et al (2019) A morphological classification for vocal cord leukoplakia. Braz Otorhinolaryngol 85:588–596
Pietrszewska W, Morawska J, Rosiak O et al (2021) Vocal cord leukoplakia: which of the classifications of white light and narrow band imaging most accurately predicts laryngeal cancer transformation? Proposition for a Diagnostic Algorithm Cancers 13:3273
Young CK, Lin WN, Lee LY et al (2015) Laryngoscopic characteristics in vocal leukoplakia: inter-rater reliability and correlation with histology grading. Laryngoscope 125:62–66
Lee DH, Yoon TM, Lee JK et al (2015) Predictive factors of recurrence and malignant transformation in vocal cord leukoplakia. Eur Arch Otorhinolaryngol 272:1719–1724
Andrea M, Dias O, Santos A (1995) Contact endoscopy of the vocal cord: normal and pathological patterns. Acta Otolaryngol 115:314–326
Andrea M, Dias O, Santos A (1995) Contact endoscopy during microlaryngeal surgery: a new technique for endoscopic examination of the larynx. Ann Otol Rhinol Laryngol 104:333–339
Mishra A, Nilakantan A, Datta R et al (2012) Contact endoscopy - a promising tool for evaluation of laryngeal mucosal lesions. J Laryngol Voice 2:53–59
Pavlidis P, Gouveris H, Anogeianaki A, Koutsonikolas D, Anogianakis G, Kekes G (2013) Age-related changes in electrogustometry thresholds, tongue tip vascularization, density, and form of the fungiform papillae in humans. Chem Senses 38:35–43
Pavlidis P, Gouveris H, Kekes G, Maurer J (2014) Electrogustometry thresholds, tongue tip vascularization, and density and morphology of the fungiform papillae in diabetes. B-ENT 10:271–278
Pavlidis P, Cámara RJA, Kekes G, Gouveris H (2018) Bilateral taste disorders in patients with Ramsay Hunt syndrome and Bell palsy. Ann Neurol 83:807–815
Pavlidis P, Gouveris H, Kekes G (2017) Electrogustometry thresholds, tongue tip vascularization, density, and form of the fungiform papillae following smoking cessation. Chem Senses 42:419–423
Arens C, Malzahn K, Dias O et al (1999) Endoscopic imaging techniques in the diagnosis of laryngeal carcinoma and its precursor lesions. Laryngorhinootologie 78:685–691
Ni XG, He S, Xu ZG et al (2011) Endoscopic diagnosis of laryngeal cancer and precancerous lesions by narrow band imaging. J Laryngol Otol 125:288–296
Arens C, Piazza C, Andrea M et al (2016) Proposal for a descriptive guideline of vascular changes in lesions of the vocal cords by the committee on endoscopic laryngeal imaging of the European Laryngological Society. Eur Arch Otorhinolaryngol 273:1207–1214
Gale N, Poljak M, Zidar N (2017) Update from the 4th Edition of the World Health Organization Classification of Head and Neck Tumours What is New in the WHO Blue Book for Tumours of the Hypopharynx Larynx Trachea and Parapharyngeal Space. Head and Neck Pathol 11: 23–32
Piazza C, Del Bon F, Peretti G, Nicolai P (2012) Narrow band imaging in endoscopic evaluation of the larynx. Curr Opin Otolaryngol Head Neck Surg 20:472–476
Postma GN, Courey MS, Ossoff RH (1998) Microvascular lesions of the true vocal cord. Ann Otol Rhinol Laryngol 107:472–476
Lukes P, Zabrodsky M, Lukesova E, Chovanec M et al (2014) The role of NBI HDTV magnifying endoscopy in the prehistologic diagnosis of laryngeal papillomatosis and spinocellular cancer. Biomed Res Int 2014:85486
Baletic N, Petrovic Z, Pendjer I, Malicevic H (2004) Autofluorescent diagnostics in laryngeal pathology. Eur Arch Otorhinolaryngol 261:233–237
Paczona R, Temam S, Janot F, Marandas P et al (2003) Autofluorescence videoendoscopy for photodiagnosis of head and neck squamous cell carcinoma. Eur Arch Otorhinolaryngol 260:544–548
Laccourreye O, Veivers FD, Hans S et al (2002) Metachronous second primary cancers after successful partial laryngectomy for invasive squamous cell carcinoma of the true vocal cord. Ann Otol Rhinol Laryngol 111:204–209
Warnecke A, Averbeck T, Leinung M et al (2010) Contact endoscopy for the evaluation of the pharyngeal and laryngeal mucosa. Laryngscope 120:253–258
Pak MW, To KF Leung SF, van Hasselt CA (2002) In vivo diagnosis of persistent and recurrent nasopharyngeal carcinoma by contact endoscopy. Laryngoscope 112: 1459-1466
Cikojevic D, Gluncic I, Pesustik-Pisac V (2008) Comparison of contact endoscopy and frozen section histopathology in the intra-operative diagnosis of laryngeal pathology. J Laryngol Otol 122:836–839
Acknowledgements
The authors wish to thank Mrs. Aikaterini Tsipropoulou for her excellent assistance in reviewing the literature.
Funding
Open access funding provided by HEAL-Link Greece. The authors received no funding.
Author information
Authors and Affiliations
Contributions
PP and HG contributed to conceptualization. AC contributed to data searching. PP, VST, IK, and AC contributed to data curation. HG contributed to formal analysis and visualization. PP, VST, and AC contributed to methodology. PP,CM and VST contributed to writing – the original draft. HG contributed to writing – review, and editing.
Corresponding author
Ethics declarations
Conflict of interest
All authors declare that they have no conflict of interest.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards. This study was performed after approval of the local ethics committee(Papanikolaou General Hospital, Thessaloniki, Nr. 72–2204202).
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Pavlidis, P., Tseriotis, V.S., Matthias, C. et al. Contact Endoscopic Surface Vascular and Epithelial Morphology in Leukoplakia and Carcinoma of the Vocal Cords. Indian J Otolaryngol Head Neck Surg 76, 462–468 (2024). https://doi.org/10.1007/s12070-023-04183-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12070-023-04183-5