Abstract
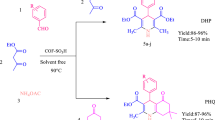
Recent research underscores the significance of metal-organic frameworks as catalysts, owing to their structural adaptability, substantial surface areas, adjustable pore dimensions, and customizable catalytic sites. Using Friedländer synthesis, we evaluated the catalytic potential of three synthesized metal-organic framework materials, MIL-53(Al), MIL-101(Cr), and MOF-5(Zn), in quinoline derivative synthesis. MIL-53(Al) outperformed MIL-101(Cr) and MOF-5(Zn), highlighting the vital role of Lewis acidic sites (Al3+) in quinoline production. Potentiometric titration analyses revealed MIL-53(Al)'s superior Lewis acid strength. Reaction optimization involved varying temperatures, catalyst loading, reaction duration, and solvents. MIL-53(Al) exhibited four-cycle recyclability. Mechanistic insights underscored Lewis acid strength and the significance of sites. The Al-based catalyst proficiently facilitated Friedlander synthesis, yielding enhanced conversion and considerable physiologically active quinoline yields. The findings offer insights into diverse catalytic strategies and demonstrate the adaptability of metal-organic framework materials in varied chemical reactions.
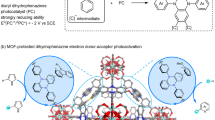
Graphical Abstract
The Al-based Lewis acid MOF catalyst MIL-53(Al) efficiently facilitated the Friedlander synthesis, resulting in improved conversion and significant yields of physiologically active quinolines. These findings provide insights into versatile catalytic strategies and showcase the adaptability of MOFs in diverse chemical reactions.






Similar content being viewed by others
References
Pe E, Samadi A, Carreiras C and Soriano E 2009 Recent Advances in the Friedlander Chem. Rev. 109 2652
Akbari J, Heydari A, Kalhor H R and Kohair S A 2010 Sulfonic acid functionalized ionic liquid in combinatorial approach, a recyclable and water tolerant-acidic catalyst for one-pot friedlander quinoline synthesis J. Comb. Chem. 12 137
Garella D et al., 2009 Fast, solvent-free, microwave-promoted friedländer annulation with a reusable solid catalyst Synth. Commun. 40 120
Martín-Aranda R M and Čejka J 2010 Recent advances in catalysis over mesoporous molecular sieves Top. Catal. 53 141
Bose D S and Kumar R K 2006 An efficient, high yielding protocol for the synthesis of functionalized quinolines via the tandem addition/annulation reaction of o-aminoaryl ketones with α-methylene ketones Tetrahedron Lett. 47 813
Wu J, Zhang L and Diao T N 2005 An expeditious approach to quinolines via Friedländer synthesis catalyzed by FeCl3 or Mg(ClO4)2 Synlett 17 2653
Yadav J S, Reddy B V S and Premalatha K 2004 Bi(OTf)3-catalyzed Friedländer hetero-annulation: A rapid synthesis of 2,3,4-trisubstituted quinolines Synlett 7 963
De S K and Gibbs R A 2005 A mild and efficient one-step synthesis of quinolines Tetrahedron Lett. 46 1647
Arumugam P, Karthikeyan G, Atchudan R, Muralidharan D and Perumal P T 2005 A simple, efficient and solvent-free protocol for the friedländer synthesis of quinolines by using SnCl2·2H2O Chem. Lett. 34 314
Kumar D et al., 2005 In(OTf)3-catalyzed synthesis of 2-styryl quinolines: Scope and limitations of metal Lewis acids for tandem Friedländer annulation-Knoevenagel condensation RSC Adv. 5 2920
Wang Z 2010 Comprehensive OrganicName Reactions and Reagents (John Wiley: Hoboken, NJ, USA)
Meenu P C, Datta S P, Dinda S, Singh S A, Chakraborty C and Roy S 2021 A compendium on metal organic framework materials and their derivatives as electrocatalyst for methanol oxidation reaction Mol. Catal. 510 111710
Lawrence A S, Sivakumar B and Dhakshinamoorthy A 2022 Detecting Lewis acid sites in metal-organic frameworks by density functional theory Mol. Catal. 517 112042
Dhakshinamoorthy A and Garcia H 2012 Catalysis by metal nanoparticles embedded on metal–organic frameworks Chem. Soc. Rev. 41 5262
Dalapati R et al., 2016 A highly stable dimethyl-functionalized Ce(IV)-based UiO-66 metal-organic framework material for gas sorption and redox catalysis CrystEngComm 18 7855
Krishna B, Payra S and Roy S 2022 Synthesis of dihydropyrimidinones via multicomponent reaction route over acid functionalized Metal-Organic framework catalysts J. Colloid Interface Sci. 607 729
Hall J N and Bollini P 2020 Metal-Organic Framework MIL-100 Catalyzed cetalization of Benzaldehyde with Methanol: Lewis or Brønsted Acid Catalysis ACS Catal. 10 3750
Anbu N and Dhakshinamoorthy A 2018 Regioselective ring opening of styrene oxide by carbon nucleophiles catalyzed by metal-organic frameworks under solvent-free conditions J. Ind. Eng. Chem. 58 9
Payra S and Roy S 2021 From Trash to Treasure: Probing Cycloaddition and Photocatalytic Reduction of CO2 over Cerium-Based Metal-Organic Frameworks J. Phys. Chem. C 125 8497
Zhang X N, Liu L, Han Z B, Gao M L and Yuan D Q 2015 A dual-functional Cd(II)-organic-framework demonstrating selective sensing of Zn2+ and Fe3+ ions exclusively and size-selective catalysis towards cyanosilylation RSC Adv. 5 10119
Dhakshinamoorthy A, Alvaro M and Garcia H 2011 Metal-organic frameworks as heterogeneous catalysts for oxidation reactions Catal. Sci. Technol. 1 856
Dhakshinamoorthy A, Asiri A M and Garcia H 2016 Metal-Organic Frameworks as Catalysts for Oxidation Reactions Chem. - A Eur. J. 22 8012
Payra S et al., 2022 Photo- and Electrocatalytic Reduction of CO2 over Metal-Organic Frameworks and Their Derived Oxides: A Correlation of the Reaction Mechanism with the Electronic Structure Inorg. Chem. 61 2476
Payra S, Devaraj N, Tarafder K and Roy S 2022 Unprecedented Electroreduction of CO2 over Metal Organic Framework-Derived Intermetallic Nano-Alloy Cu0.85Ni0.15/C ACS Appl. Energy Mater. 5 4945
Li H, Yang T and Fang Z 2018 Biomass-derived mesoporous Hf-containing hybrid for efficient Meerwein-Ponndorf-Verley reduction at low temperatures Appl. Catal. B Environ. 227 79
Payra S, Kanungo S and Roy S 2022 Controlling C-C coupling in electrocatalytic reduction of CO2 over Cu1−xZnx/C Nanoscale 14 13352
Dhakshinamoorthy A, Asiri A M and Garcia H 2015 Metal-organic frameworks catalyzed C-C and C-heteroatom coupling reactions Chem. Soc. Rev. 44 1922
Park J, Li J-R, Chen Y-P, Yu J, Yakovenko A A, Wang Z U, et al. 2012 A versatile metal-organic framework for carbon dioxide capture and ooperative catalysis Chem. Commun. 48 9995
Loiseau T, Serre C, Huguenard C, Fink G, Taulelle F, Henry M, et al. 2004 A rationale for the large breathing of the porous aluminum terephthalate (MIL-53) upon hydration Chem. Eur. J. 10 1373
Lebedev OI, Millange F, Serre C, Van Tendeloo G and Férey G 2005 First Direct Imaging of Giant Pores of the Metal−Organic Framework MIL-101 Chem. Mater. 17 6525
Qing L, Liqi N, Shudong Z, Mengna T, Yao S and Yi H 2013 Adsorption of Carbon Dioxide by MIL-101(Cr): Regeneration Conditions and Influence of Flue Gas Contaminants Sci. Rep. 3 2916
Wu C-M, Rathi M, Ahrenkiel S P, Koodali R T and Wang Z 2013 Facile synthesis of MOF-5 confined in SBA-15 hybrid material with enhanced hydro stability Chem. Commun. 49 1223
Salmi L D, Heikkilä M J, Puukilainen E, Sajavaara T, Grosso D and Ritala M 2013 Studies on atomiclayer deposition of MOF-5 thinfilms Micropor. Mesopor. Mater. 182 147
Li B et al. 2015 Metal-organic framework based upon the synergy of a Brønsted acid framework and Lewis acid centers as a highly efficient heterogeneous catalyst for fixed-bed reactions J. Am. Chem. Soc. 137 4243
López-Sanz J, Pérez-Mayoral E, Procházková D, Martín-Aranda R M and López-Peinado A J 2010 Zeolites promoting quinoline synthesis via Friedländer reaction Top. Catal. 53 1430
Arcadi A, Chiarini M, Di Giuseppe S and Marinelli F 2015 A new green approach to the Friedländer synthesis of quinolines Synlett 2 203
Pérez-Mayoral E and Čejka J 2011 [Cu3(BTC)2]: A Metal-Organic Framework Catalyst for the Friedländer Reaction ChemCatChem 3 157
Villabrille P, Vázquez P, Blanco M and Cáceres C 2002 Equilibrium adsorption of molybdosilicic acid solutions on carbon and silica: Basic studies for the preparation of ecofriendly acidic catalysts J. Colloid Interface Sci. 251 151
Pizzio L R, Vázquez P G, Cáceres C V and Blanco M N 2003 Supported Keggin type heteropolycompounds for ecofriendly reactions Appl. Catal. A Gen. 256 125
Roy S 2020 Photocatalytic Materials for Reduction of Nitroarenes and Nitrates J. Phys. Chem. C 52 28345
Acknowledgment
B. Krishna would like to thank Adama India Pvt. Ltd. for financial support. Both the authors acknowledge BITS Pilani Hyderabad Campus.
Funding
Birla Institute of Technology and Science, Pilani.
Author information
Authors and Affiliations
Contributions
Bandarupalli Krishna: Investigation, Data curation, Validation, Writing-original draft. Sounak Roy: Conceptualization, Writing, Review & editing.
Corresponding author
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Krishna, B., Roy, S. Synthesis of Quinolines from 2-amino aryl ketones: Probing the Lewis Acid Sites of Metal-Organic Framework Catalyst. J Chem Sci 136, 17 (2024). https://doi.org/10.1007/s12039-024-02257-7
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12039-024-02257-7