Abstract
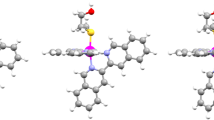
Four new ternary Oxovanadium(IV) complexes, [VO(L)(B1-4)](acac), where, L = (E)-2- (2-hydroxybenzylideneamino)-5-guanidinopentanoic acid (Argininesalisylidene) and B were 1H-imidazo-[4,5-f][1,10]-phenanthroline (B1) (1), 2-phenyl-1H-imidazo [4,5-f][1,10] phenanthroline (B2) (2), 2-(naphthalen-1-yl)-1H-imidazo [4,5-f][1,10] phenanthroline (B3) (3) or 1-(pyren-2-yl)-1H-imidazo[4,5-f][1,10] phenanthroline (B4) (4) were synthesized, characterized and photocytotoxicity in A549 cell were studied in dark and visible light (400–700 nm, 10 J cm−2). Photochemistry of the complexes (1-4) related to the generation of singlet oxygen (1O2) was studied in detail. The presence of triplet excited state in the complexes was probed from perylene assay. Photo-activated generation of singlet oxygen from the complexes was probed by DPBF assay and the degree of photo-cytotoxicity of the complexes was related to their ability in photo-activated 1O2 generation. Complexes 4 exhibited remarkable photocytotoxicity in A549 cells with photo index (PI) ~ 16 in visible light (400–700 nm, 10 J cm−2) (IC50, 3.81 µM), while complexes were almost less-toxic in dark (IC50, 59.7 µM). Intracellular ROS generation was studied from 2′,7′-dichlorofluorescein diacetate (DCFH-DA). Annexin V-FITC/PI assay suggested apoptotic nature of cell death. Overall, the remarkable photocytotoxic efficacy of the new Oxovanadium(IV) complexes (1-4) making them the potential photochemotherapeutic agents in lieu of porphyrin-based PDT agents.
Graphical abstract







Similar content being viewed by others
References
Dolmans E J G J D, Fukumura D and Jain K R 2003 Photodynamic therapy for cancer Nat. Rev. Cancer 3 380
Wait S, Han D, Muthu V, Oliver K, Chrostowski S, Florindi F, et al. 2017 Towards sustainable cancer care: reducing inefficiencies, improving outcomes—a policy report from the All Can initiative J. Cancer Pol. 13 47
Abbas Z and Rehman S 2018 An Overview of Cancer Treatment Modalities Neoplasm. https://doi.org/10.5772/intechopen.76558.
Bonnett R 2000 Chemical Aspects of Photodynamic Therapy (Gordon & Breach: London, UK)
Henderson B W, Busch T M, Vaughan L A, Frawley N P, Babich D, Sosa T A, et al. 2000 Photofrin photodynamic therapy can significantly deplete or preserve oxygenation in human basal cell carcinomas during treatment, depending on fluence rate Cancer Res. 60 525
DeRosa M C and Crutchley R 2002 Coordination chemistry reviews J. Coord. Chem. Rev. 233 351
Dulal Musib, Raza M K, Kundu S and Roy M 2018 Modulating In Vitro photodynamic activities of copper (II) complexes Eur J. Inorg. Chem. 19 2011
Liu H K, Berners-Price S J, Wang F, Parkinson J A, Xu J, Bella J and Sadler P J 2006 Diversity in guanine-selective DNA binding modes for an organometallic ruthenium arene complex Angew. Chem. 118 8333
Zhang C X and Lippard S J 2003 New metal complexes as potential therapeutics Curr Opin. Chem. Biol. 7 481
Huang Z 2005 A review of progress in clinical photodynamic therapy Technol Cancer Res. Treat. 4 283
Dhar S, Liu Z, Thomale J, Dai H and Lippard S J 2008 Targeted single-wall carbon nanotube-mediated Pt(IV) prodrug delivery using folate as a homing device J. Am. Chem. Soc. 130 11467
Mackay F S, Woods J A, Heringova P, Kasparkova J, Pizarro A M, Moggach S A, et al. 2007 A potent cytotoxic photoactivated platinum complex Proc Natl. Acad. Sci. USA 104 20743
Wei W H, Wang Z, Mizuno T, Cortez C, Fu L, Sirisawad M, Naumovski L, Magda D and Sessler J L 2006 New polyethyleneglycol-functionalized texaphyrins: synthesis and in vitro biological studies Dalton Trans. 1934
Sternberg E D, Dolphin D and Bruckner C 1998 Porphyrin-based photosensitizers for use in photodynamic therapy Tetrahedron 54 4151
Lb L and Rc L 2009 Effect of drug-light interval on the mode of action of Photofrin photodynamic therapy in a mouse tumor model Lasers Med Sci. 24 597
Bonnett R 1995 Photosensitizers of the porphyrin and phthalocyanine series for photodynamic therapy Chem. Soc. Rev. 24 19
Moriwaki S I, Misawa J, Yoshinari Y, Yamada I, Takigawa M and Tokura Y 2001 Analysis of photosensitivity in Japanese cancer-bearing patients receiving photodynamic therapy with porfimer sodium (Photofrin) Photodermatol Photoimmunol. Photomed. 5 241
Musib D, Raza M K, Pal M and Roy M 2020 A red light‐activable MnI(CO)3‐functionalized gold nanocomposite as the anticancer prodrug with theranostic potential. Appl. Organomet. e6110
Rosenberg B, VamCamp L, Trosko J E and Mansour V H 1969 Platinum compounds: a new class of potent antitumour agents Nature 222 385
Musib D, Raza M K, Martina K and Roy M 2019 Mn (I)- based photoCORMS for trackable, visible light-induced CO release and photocytototoxicity to cancer cells Polyhedron 172 125
Chanu S B, Raza M K, Banerjee S, Mina P R, Musib D and Roy M 2019 ROS dependent antitumour activity of photo-activated iron (III) complexes of amino acids J. Chem. Sci. 131 9
(a) Jungwirth U, Kowol C R, Keppler, Hartinger C G, Berger W and Heffeter P 2011 Anticancer activity of metal complexes: involvement of redox processes Antioxid Redox Signal. 15 1085; (b) Prasad P, Sasmal P K, Majumdar R, Dighe R R and Chakravarty A R 2010 Photocytotoxicity and near-IR light DNA cleavage activity of oxovanadium(IV) Schiff base complexes having phenanthroline bases Inorg. Chim. Acta 333 2743
Balaji B, Banik B, Sasmal P K, Maity B, Majumdar R, Dighe R R and Chakravarty A R 2011 Ferrocene-Conjugated Oxidovanadium(IV) Complexes as Potent Near-IR Light Photocytotoxic Agents Eur. J. Med. Chem. 2012 126
(a) Saha S, Majumdar R, Roy M, Dighe R R and Chakravarty A R 2009 An iron complex of dipyridophenazine as a potent photocytotoxic agent in visible light Inorg. Chem. 48 2652; (b) Abakumova O Y, Podobed O V, Belayeva N F and Tochilkin A I 2013 Biomed. Khim. 59 305
Raza M K, Gautam S, Garai A, Mitra K, Kondaiah P and Chakravarty A R 2017 Monofunctional BODIPY- appended imidazoplatin for cellular imaging and mitochondria-targeted photocytotoxicity Chem. Chem. 56 11019
Kesavan P E, Pandey V, Raza M K, Mori S and Gupta I 2019 Water soluble thioglycosylated BODIPYs for mitochondria targeted cytotoxicity Bioorg. Chem. 91 103139
(a) Ta S, Ghosh M, Ghosh K, Brandao P, Felix V, Hira S K, Manna P P and Das D 2019 Exploring Anticancer and (Bio)catalytic activities of new Oxovanadium(V), Dioxomolybdenum(VI), and Copper(II) complexes of amide–imine conjugates ACS Appl. Bio. Mater. 2 2802; (b) Banerjee S, Pant I, Khan I, Prasad P, Hussain A, Kondaiah P and Chakravarty A R 2015 Remarkable enhancement in photocytotoxicity and hydrolytic stability of curcumin on binding to an oxovanadium(iv) moiety Dalton Trans. 44 4108
(a) Bhattacharyya A, Dixit A, Mitra K, Banerjee S, Karande A A and Chakravarty A R 2015 BODIPY appended copper(II) complexes of curcumin showing mitochondria targeted remarkable photocytotoxicity in visible light Med. Chem. Comm. 6 846; (b) Kostova I 2009 Titanium and vanadium complexes as anticancer agents. Anticancer agents Anticancer Agent. Med. Chem. 9 827
Lohar S, Pal S, Sen B, Mukherjee M, Banerjee S and Chattopadhyay P 2014 Selective and sensitive turn-on chemosensor for arsenite Ion at the ppb level in aqueous media applicable in cell staining Anal. Chem. 86 11357
Jung Y and Lippard S J 2007 Direct cellular responses to platinum-induced DNA damage Chem Rev. 107 1387
(a) Woodburn K 2001 Chemical aspects of photodynamic therapy by Raymond bonnett University of London: Gordon and Breach Science Publishers: London and Newark 2000 J. Am. Chem. Soc. 123 3622; (b) Sessler J L and Miller R A 2000 Texaphyrins: new drugs with diverse clinical applications in radiation and photodynamic therapy Biochem. Pharmacol. 59 733
(a) Chifotides H T and Dunbar K R 2005 Interactions of metal−metal-bonded antitumor active complexes with DNA fragments and DNA Acc. Chem. Res. 38 146; (b) Sgambellone M A, David A, Garner R N, Dunbar K R and Turro C J 2013 Cellular toxicity induced by the photorelease of a caged bioactive molecule: Design of a potential dual-action Ru(II) complex Am. Chem. Soc. 135 11274
(a) Canelon I R and Sadler P J 2013 Next-generation metal anticancer complexes: multitargeting via redox modulation Inorg. Chem. 52 12276; (b) Smith N A and Sadler P J 2013 Photoactivatable metal complexes: from theory to applications in biotechnology and medicine Phil. Trans. Royal. Soc. A 371 20120519; (c) Schatzschneider U 2010 Photoactivated biological activity of transition‐metal complexes Eur. J. Inorg. Chem. 2010 1451; (d) Fry N L and Mascharak P K 2011 Photoactive ruthenium nitrosyls as NO donors: how to sensitize them toward visible light Acc. Chem. Res. 44 289
(a) Wachter E, Heidary D K, Howerton B S, Parkin S and Glazer E C 2012 Light-activated ruthenium complexes photobind DNA and are cytotoxic in the photodynamic therapy window Chem. Commun. 48 9649; (b) Higgins S L H, Tucker A J, Winkel B S J and Brewer K J 2012 Metal to ligand charge transfer induced DNA photobinding in a Ru(ii)–Pt(ii) supramolecule using red light in the therapeutic window: a new mechanism for DNA modification Chem. Commun. 48 67
Jin Y and Cowan J A 2005 DNA cleavage by copper−ATCUN complexes Factors influencing cleavage mechanism and linearization of dsDNA J. Am. Chem. Soc. 127 8408
Sasmal PK, Patra AK, Nethaji M and Chakravarty A R 2007 DNA cleavage by new oxovanadium (IV) complexes of N-salicylidene α-amino acids and phenanthroline bases in the photodynamic therapy window Inorg Chem. 46 11112
Mertz W 1981 The essential trace elements Science 213 1332
Macara I G 1980 Vanadium —an element in search of a role Trends Biochem. Sci. 5 92
Trevino S, Diaz A, Sanchez-Lara E, Sanchez-Gaytan B L, Perez-Aguilar J M and Gonzalez-Vergara E 2019 Vanadium in biological action: chemical, pharmacological aspects, and metabolic implications in diabetes mellitus Drug Discov. Today 188 68
Crans D C and Tracey A S 1998 The chemistry of vanadium in aqueous and nonaqueous solution J. Am. Chem. Soc. 711 2
(a) Samanta S, Ghosh D, Mukhopadhyay S, Endo A, Weakley T J R and Chaudhury M 2003 Oxovanadium(IV) and -(V) Complexes of dithiocarbazate-based tridentate schiff base ligands: syntheses, structure, and photochemical reactivity of compounds involving imidazole derivatives as coligands Inorg. Chem. 42 1508; (b) Evangelou A M 2002 Vanadium in cancer treatment Crit. Rev. Oncol. Hematol. 42 249
Crans D C, Zhang B, Gaidamauskas E, Keramidas A D, Willsky G R and Roberts C R 2010 Is vanadate reduced by thiols under biological conditions? Changing the redox potential of V(V)/V(IV) by complexation in aqueous solution Inorg. Chem. 49 4245
Sun Y, Zheng Y, Lei W, Zhou Q, Hou Y, Zhang B and Wang X 2012 Oxovanadium(IV) based hypocrellin B complexes with enhanced photodynamic activity Dalton Trans. 41 651
Goswami T K, Gadadhar S, Roy M, Nethaji M, Karande A A and Chakravarty A R 2012 Ferrocene-conjugated copper(II) complexes of l-methionine and phenanthroline bases: synthesis structure, and photocytotoxic activity Organometallics 31 3010
Ross A, Soares D C, Covelli D, Pannecouque C, Budd L, Collins A and Sadler P J 2010 Oxovanadium(IV) cyclam and bicyclam complexes: potential CXCR4 receptor antagonists Inorg. Chem. 49 1122
Thompson K H and Orvig C 2001 Coordination chemistry of vanadium in metallopharmaceutical candidate compounds Coord. Chem. Rev. 219 1033
Adachi Y, Yoshida J, Kodera Y, Katoh A, Takada J and Sakurai H 2006 Bis(allixinato)oxovanadium(IV) complex is a potent antidiabetic agent: studies on structure−activity relationship for a series of hydroxypyrone−vanadium complexes J. Med. Chem. 49 3251
Sasmal P K, Saha S, Majumdar R, De S, Dighe R R and Chakravarty AR 2010 Oxovanadium(IV) complexes of phenanthroline bases: the dipyridophenazine complex as a near-IR photocytotoxic agent Dalton Trans. 39 2147
Adachi Y, Yoshida J, Kodera Y, Katoh A, Takada J and Sakurai H 2006 Bis(allixinato)oxovanadium(IV) is a potent antidiabetic agent: studies on structure−activity relationship for a series of hydroxypyrone−vanadium complexes J. Med. Chem. 49 3251
Fan S H, Zhang A G, Ju C C, Gao L H and Wang K Z 2010 A Triphenylamine-Grafted Imidazo[4,5-f][1,10]phenanthroline Ruthenium(II) complex: acid−base and photoelectric properties Inorg. Chem. 49 3752
Banerjee S, Prasad P, Khan I, Hussain A, Kondaiah P and Chakravarty A R 2014 Mitochondria targeting photocytotoxic oxidovanadium(IV) complexes of curcumin and (acridinyl)dipyridophenazine in visible light Z. Anorg. Allg. Chem. 640 1195
Fedorova O A, Shepel N E, Tokarev S D, Lukovskaya E V, Sotnikova Y A, Moiseeva A A, et al. 2019 Intramolecular electron transfer in Cu(II) complexes with aryl-imidazo-1,10-phenanthroline derivatives: experimental and quantum chemical calculation studies New J. Chem. 43 2817
Prasad P, Pant I, Khan I, Kondaiah P and Chakravarty A R 2014 Mitochondria targeted photo-induced anticancer activity of Oxidovanadium(IV) complexes of curcumin in visible light Eur. J. Inorg. Chem. 14 2420
Wang J, Xu S, Zhao F, Xia H and Wang Y 2016 Computational and spectroscopic studies of the imidazole-fused phenanthroline derivatives containing phenyl, naphthyl, and anthryl groups J. Mol. Struct. 1108 46
Bhat S S, Kumbhar A A, Heptullah H, Khan A A, Gobre V V, Gejji S P and Puranik V G 2011 Synthesis, electronic structure, DNA and protein binding, DNA cleavage, and anticancer activity of fluorophore-labeled copper(II) complexes Inorg. Chem. 50 545
Ji S, Wu W, Wu W, Guo H, Yang Q, Wang Q, et al. 2010 Synthesis of polypyridyl ruthenium complexes with 2-(1-aryl)-1H-imidazo[4,5-f]-1,10-phenanthroline ligand and its application for luminescent oxygen sensing Front. Chem. China 5 193
Mao J, Li N, Li H and Hu X 2006 Novel Schiff base complexes as catalysts in aerobic selective oxidation of β-isophorone J. Mol. A Chem. 258 178
Rossi L M, Silva P R, Vono L L R, Fernandes A U, Tada D B and Baptista M S 2008 Protoporphyrin IX nanoparticle carrier: preparation, optical properties, and singlet oxygen generation Langmuir 24 12534
Raza M K, Mitra K, Shettar A, Basu U, Kondaiah P and Chakravarty AR 2016 Photoactive platinum(ii) β-diketonates as dual action anticancer agents Dalton Trans. 45 13234
Hoebeke M and Damoiseau X 2002 Determination of the singlet oxygen quantum yield of bacteriochlorina: a comparative study in phosphate buffer and aqueous dispersion of dimiristoyl-l-α-phosphatidylcholine liposomes Photochem. Photobiol. Sci. 1 283
Wu L, Yang L, Huang J, Zhang L, Weng X, Zhang X, et al. 2009 Cationic ester porphyrins cause high levels of phototoxicity in tumor cells and induction of apoptosis in HeLa cells Chem. Biodivers. 6 1066
Paul A, Mistri S, Bhunia A, Manna S, Puschmann H and Manna SC 2016 Synthesis, crystal structure, DFT/TDDFT calculation, photophysical properties and DNA binding studies of morpholino moiety ligand based two Cu(ii) complexes in combination with carboxylates RSC Adv. 6 60487
(a) Stratmann R E, Scuseria G E and Frisch M J 1998 An efficient implementation of time-dependent density-functional theory for the calculation of excitation energies of large molecules J. Chem. Phys. 109 8218; (b) Casida M E, Jamorski C, Casida K C and Salahub D R 1998 Molecular excitation energies to high-lying bound states from time-dependent density-functional response theory: Characterization and correction of the time-dependent local density approximation ionization threshold J. Chem. Phys. 108 4439
Wei Y, Zheng M, Zhou Q, Zhou X and Liu S 2018 Application of a bodipy–C70 dyad in triplet–triplet annihilation upconversion of perylene as a metal-free photosensitizer Org. Biomol. Chem. 16 5598
Ye C, Gray V and Martensson J 2019 Annihilation versus excimer formation by the triplet pair in triplet-triplet annihilation photon upconversion J. Am. Chem. Soc. 141 9578
(a) Bhattacharyya A, Dixit A, Banerjee S, Roy B, Kumar A, Karande A A and Chakravarty A R 2016 BODIPY appended copper(ii) complexes for cellular imaging and singlet oxygen mediated anticancer activity in visible light RSC Adv. 6 104474; (b) Gandra N, Frank A T, Gendre O L, Sawwan N, Aebisher D, Liebman J F, Houk K N, Greerb A and Gaoa R 2006 Possible singlet oxygen generation from the photolysis of indigo dyes in methanol, DMSO, water, and ionic liquid, 1-butyl-3-methylimidazolium tetrafluoroborate Tetrahedron 62 10771
Musib D, Pal M, Raza MK and Roy M 2020 Photo-physical, theoretical and photo-cytotoxic evaluation of a new class of lanthanide (III)–curcumin/diketone complexes for PDT application Dalton Trans. 49 10786
Acknowledgements
We gratefully thank the Board of Research in Nuclear Science (BRNS), Mumbai (37(2)/14/18/2017-BRNS) for funding. We also would like to thank the National Institute of Technology, Manipur, for providing a research facility to carry out the work. We sincerely thank Prof. Akhil R Chakravarty, IISc Bangalore for providing support to carry out the project. We gratefully thank the Central Instruments Facility, IIT Guwahati for providing the EPR data.
Author information
Authors and Affiliations
Corresponding author
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Sanasam, B., Raza, M.K., Musib, D. et al. Photochemical and photocytotoxic evaluation of new Oxovanadium (IV) complexes in photodynamic application. J Chem Sci 133, 42 (2021). https://doi.org/10.1007/s12039-021-01896-4
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12039-021-01896-4