Abstract
Several amino acid-based photo-active monomeric iron(III) complexes of the general formula, \([\hbox {Fe(L)}_{2}]^{-}\), where \(\hbox {L} = \) Schiff base ligands (salisalidene arginine, salicylidenetryptophan, 3,5-di-tert-butyl benzalidine arginine and salicylidene tryptophan) were synthesized, characterized and explored for photo-activated anticancer activity to Chang Liver Cells, HeLa and MCF-7 cells. Complexes exhibited remarkable photo-cytotoxicity with \(\hbox {IC}_{{50}}\) value to the extent of \(0.7\, \upmu \hbox {M}\) to Chang Liver Cells in visible light and there was a 40-fold enhancement in cytotoxicity in comparison to the cytotoxicity in dark. Complexes were non-toxic to MCF-10A (normal cells) in dark and visible light (\(\hbox {IC}_{50 }> 100 \, \upmu \hbox {M}\) in dark; \(\hbox {IC}_{50 }> 80 \, \upmu \hbox {M}\) in visible light) signifying target-specific nature of the anti-tumour activity of the complexes. Increased ROS concentration, as probed by DCFDA assay, in the cancer cells was responsible for apoptotic cell death. Decarboxylation or phenolate-Fe(III) charge transfer of photo-activated iron(III) complexes generating \(^{\bullet }\)OH radicals (ROS) were responsible for the apoptosis. Overall, the tumour-selective photo-activated anticancer activity of the amino acid-based iron(III) complexes have shown a promising aspect in developing iron-based photo-chemotherapeutics as the next generation PDT agents.
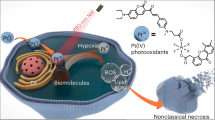
Graphical abstract
Monomeric iron(III) complexes are explored for photo-activated antitumour activity. Photodecarboxylation or photo-induced charge transfer of phenolate-\(\hbox {O}{\rightarrow }\hbox {Fe(III)}\) has led to the generation of hydroxyl radicals causing apoptotic cell death.









Similar content being viewed by others
Change history
08 February 2019
Unfortunately, Figure 1 was published incorrectly in the original version. The correct figure 1 is provided below.
References
(a) Wang X, Wang X and Guo Z 2018 Metal-involved theranostics: An emerging strategy for fighting Alzheimer’s disease Coord. Chem. Rev. 362 72; (b) Mital M and Zoira Z 2018 Biological applications of Ru(II) polypyridyl complexes Coord. Chem. Rev. 375 434; (c) Rosenberg B, VanCamp L and Krigas T 1965 Inhibition of cell division in Escherichia coli by electrolysis products from a platinum electrode Nature 205 698
Zamora A, Vigueras G, Rodríguez V, Santana M D and Ruiz J 2018 Cyclometalated iridium(III) luminescent complexes in therapy and phototherapy Coord. Chem. Rev. 360 34
(a) Marzenell P, Hagen H, Sellner L, Zenz T, Grinyte R, Pavlov V, Daum S and Mokhir A 2015 Aminoferrocene-based prodrugs and their effects on human normal and cancer cells as well as bacterial cells J. Med. Chem. 56 6935; (b) Reshetnikov V, Daum S and Mokhir A 2017 Cancer-specific, intracellular, reductive activation of anticancer Pt prodrugs Chem. Eur. J. 23 5678; (c) Fricker S P 2007 Metal based drugs: from serendipity to design Dalton Trans. 43 4903
(a) Romero-Caneló I and Sadler P J 2013 Next generation metal anticancer complexes: Multi-targeting via redox modulation Inorg. Chem. 52 12276; (b) Malik A M, Dar A O, Gull P, Wani Y M and Hashmi A A 2018 Heterocyclic Schiff base transition metal complexes in antimicrobial and anticancer chemotherapy Med. Chem. Comm. 9 409
(a) Farrer N J, Salassa L and Sadler P J 2009 Photoactivated chemotherapy (PACT): The potential of excited-state d-block metals in medicine Dalton Trans. 48 10690; (b) Zhang P and Sadler P J 2017 Redox-active metal complexes for anticancer therapy Eur. J. Inorg. Chem. 2017 1541; (c) Lakshmi S S, Geetha K, Gayathri M and Shanmugan G 2016 Synthesis, crystal structures, spectroscopic characterization and in vitro antidiabetic studies of new Schiff base Copper(II) complexes J. Chem. Sci. 128 1095
Mion G, Mari C, Da Ros T, Rubbiani R, Gasser G and Gianferrara T 2017 Towards the synthesis of new tumour targeting photosensitizers for photodynamic therapy and imagining applications Chemistry Select 2 190
Bonnet R 2000 Chemical Aspects of Photodynamic Therapy (London: Gordon & Breach) p. 57
Celli J P, Spring B Q, Rizvi I, Evans C L, Samkoe K S, Verma S, Pogue B W and Hasan T 2010 Imaging and photodynamic therapy: Mechanisms, monitoring, and optimization Chem. Rev. 110 2795
(a) Knoll J D and Turro C 2015 Control and utilization of ruthenium and rhodium metal complex excited state for photoactivated cancer therapy Coord. Chem. Rev. 282–283 110; (b) Dolmans D E J G J, Fukumura D and Jain R K 2003 Photodynamic therapy for cancer Nat. Rev. Cancer 3 380
(a) Kou J, Dou D and Yang L 2017 Porphyrin photosensitizers in photodynamic therapy and its applications Oncotarget 8 81591; (b) Jayaram DT, Ramos-Romero S, Shankar B H, Garrido C, Rubio N, Sanchez-Cid L, Gómez S B, Blanco J and Ramaiah D 2016 In vitro and in vivo demonstration of photodynamic activity and cytoplasm imaging through TPE nanoparticles ACS Chem. Biol. 11 104
Joyce L E, Aguirre J D, Angeles-Boza A M, Chouai A, Fu P K L K R and Turro C 2010 Photophysical properties, DNA photocleavage, and photocytotoxicity of a series of dppndirhodium(II,II) Complexes Inorg. Chem. 49 5371
Heinemann F, Karges J and Gasser G 2017 Critical overview of the use of Ru(II) polypyridyl complexes as photosensitizers in one-photon and two-photon photodynamic therapy Acc. Chem. Res. 50 2727
Mari C, Pierroz V, Ferrari S and Gasser G 2015 Combination of Ru(II) complexes and light: new frontiers in cancer therapy Chem. Sci. 6 2660
Raza M K, Gautam S, Garai A, Mitra K, Kondaiah P and Chakravarty A R 2017 Monofunctional BODIPY-appended imidazoplatin for cellular imaging and mitochondria-targeted photocytotoxicity Inorg. Chem. 56 11019; (b) Gupta T, Patra K A, Dhar S, Nethaji M and Chakravarty R A 2005 Effect of copper-sulfur bond on the DNA photo-cleavage activity of 2-(methylthio)ethylpyridine-2-carbaldimine copper(II) complexes J. Chem. Sci. 117 123
Zhang K Y, Ka-Shun Tso K, Louie M -W, Liu H -W and Lo K K-W 2013 A phosphorescent rhenium(I) tricarbonyl polypyridine complex appended with a fructose pendant that exhibits photocytotoxicity and enhanced uptake by breast cancer cells Organometallics 32 5098
Szaciłowski K, Macyk W, Drzewiecka-Matuszek A, Brindell M and Stochel G 2005 Bioinorganic photochemistry: Frontiers and mechanisms Chem. Rev. 105 2647
(a) Basu U, Khan I, Hussain A, Kondaiah P and Chakravarty A R 2012 Photodynamic effect in near-IR light by a remarkably photocytotoxiciron(III) cellular imaging agent Angew. Chem. Int. Ed. 51 2658; (b) Sarkar T, Banerjee S and Hussain A 2015 Significant photocytotoxic effect of an iron(III) complex of a Schiff base ligand derived from vitamin B6 and thiosemicarbazide in visible light RSC Adv. 5 29276; (c) Banerjee S, Dixit A, Maheswaramma S K, Maity B, Mukherjee S, Kumar A, Karande A and Chakravarty A R 2016 Photocytotoxic ternary copper(II) complexes of histamine Schiff base and pyridyl ligands J. Chem. Sci. 128 165
Banerjee S and Chakravarty A R 2015 Metal complexes of curcumin for cellular imaging, targeting, and photoinduced anticancer activity Acc. Chem. Res. 48 2075
(a) Musib D, Banerjee S, Garai A, Soraisam U and Roy M 2018 Synthesis, theory and in vitro photodynamic activities of new copper(II)-histidinito complexes Chemistry Select 3 2767; (b) Mukherjee N, Podder S, Mitra K, Majumdar S, Nandi D and Chakravarty A R 2018 Targeted photodynamic therapy in visible light using BODIPY-appended copper(II) complexes of a vitamin B Schiff base Dalton Trans. 47 823; (c) Mukherjee N, Podder S, Banerjee S, Majumdar S, Nandi D and Chakravarty A R 2016 Targeted photocytotoxicity by copper(II) complexes having vitamin B6 and photoactive acridine moieties Eur. J. Med. Chem. 122 497; (d) Garai A, Pant I, Bhattacharyya A, Kondaiah P and Chakravarty A R 2017 Mitochondria-targeted anticancer activity of BODIPY-appended iron(III) catecholates in red light ChemistrySelect 2 11686; (e) Musib D, Raza M K, Kundu S and Roy M 2018 Modulating in vitro photodynamic activities of copper(II) complexes Eur. J. Inorg. Chem. 2018 2011; (f) Goswami T K, Chakravarthi B V S K, Roy M, Karande A A and Chakravarty A R 2011 Ferrocene-conjugated L-tryptophan copper(II) complexes of phenanthroline bases showing DNA photocleavage activity and cytotoxicity Inorg. Chem. 50 8452
Bhattacharyya A, Dixit A, Banerjee S, Roy B, Kumar A, Karande A A and Chakravarty A R 2016 BODIPY appended copper (II) complexes for cellular imaging and singlet oxygen mediated anticancer activity in visible light RSC Adv. 6 104474
Bhattacharyya U, Kumar B, Garai A, Bhattacharyya A, Kumar A, Banerjee S, Kondaiah P and Chakravarty A R 2017 Curcumin drug stabilized in oxidovanadium(IV)-BODIPY conjugates for mitochondria-targeted photocytotoxicity Inorg. Chem. 56 12457
(a) Saha S, Patra A K, Nethaji M and Chakravarty A R 2007 Ternary iron(III) complex showing photocleavage of DNA in the PDT window Inorg. Chem. 46; (b) Saha S, Majumdar R, Roy M, Dighe R R and Chakravarty A R 2009 An iron complex of dipyridophenazine as a potent photocytotoxicagent in the visible light Inorg. Chem. 48 2652; (c) Sarkar T, Butcher R J, Banerjee S, Mukherjee S and Hussain A 2016 Visible light-induced cytotoxicity of a dinuclear iron(III) complex of curcumin with low-micromolar \(\text{IC}_{{50}}\) value in cancer cells Inorg. Chim. Acta 439 8
(a) Roy M, Bhowmick T, Santhanagopal R, Ramakumar S and Chakravarty A R 2009 Photo-induced double-strand DNA and site-specific protein cleavage activity of L-histidine (\(\upmu \)-oxo)diiron(III) complexes of heterocyclic bases Dalton Trans. 24 4671; (b) Roy M, Bhowmick T, Ramakumar S, Nethaji M and Chakravarty A R 2008 Double-strand DNA cleavage from photodecarboxylation of (\(\upmu \)-oxo)diiron(III) L-histidine complex in visible light Dalton Trans. 27 3542; (c) Roy M, Santhanagopal R and Chakravarty A R 2009 DNA binding and oxidative DNA cleavage activity of (\(\upmu \)- oxo)diiron(III) complexes in visible light Dalton Trans. 0 1024
Chanu S B, Banerjee S and Roy M 2017 Potent anticancer activity of photo-activated oxo-bridged diiron(III) complexes Eur. J. Med. Chem. 125 816
Perrin D D, Armarego W L F and Perrin D R 1980 Purification of Laboratory Chemicals (Oxford: Pergamon)
Gazi S and Ananthakrishnan R 2012 Semi-quantitative determination of hydroxyl radicals by benzoic acid hydroxylation. An analytical methodology for photo-fentonsystems Curr. Anal. Chem. 8 14
Gazi S, Rajakumar A and Singh N D P 2010 Photodegradation of organic dyes in the presence of Fe(III)-salen complex and \(\text{ H }_{{2}}\text{ O }_{{2}}\) under visible light irradiation J. Hazard. Mater. 183 894
Begum M S A, Saha S, Nethaji M and Chakravarty A R 2010 Iron(III) Schiff base complexes of arginine and lysine as netropsin mimics showing AT-selective DNA binding and photonuclease activity J. Inorg. Biochem. 104 477
Zamanifar E and Farzane F 2011 Immobilized vanadium amino acid Schiff base complexon Al-MCM-41 as catalyst for the epoxidation of allyl alcohols Reac. Kinet. Mech. Catal. 104 197
Goswami T K, Chakravarthi B V S K, Roy M, Karande A A and Chakravarty A R 2011 Ferrocene-conjugated L-tryptophan copper(II) complexes of phenanthroline bases showing DNA photocleavage activity and cytotoxicity Inorg. Chem. 50 8452
Nakamoto K 2008 Infrared and Raman Spectra of Inorganic and Coordination Compounds: Part A: Theory and Applications in Inorganic Chemistry 6\(^{{\rm th}}\) edn. (Location: John Wiley) p. 432
Frisch M J, Trucks G W and Schlegel H B 2009 Gaussian 09, Revision A. 1, Gaussian (Wallingford: USA)
Qu Y, Han B, Yu Y, Yao W, Bose S, Karlan B Y, Giuliano A E and Cui X 2015 Evaluation of MCF10A as a reliable model for normal human mammary epithelial cells PlosOne 10 e0131285
Wang H and Joseph J A 1999 Quantifying cellular oxidative stress by dichlorofluorescein assay using microplate reader Free Radicals Biol. Med. 27 612
Manfred K Eberhardt and Colina R 1988 The reaction of copper(I)-oxygen and copper(II)-ascorbic acid-oxygen with dimethyl sulfoxide. The effect of solvent J. Org. Chem. 53 1071
Wang Z, Guo Y, Liu Z, Feng X, Chen Y and Tao T 2015 Catechin as a new improving agent for a photo-Fenton-like system at near-neutral pH for the removal of inderal Photochem. Photobiol. Sci. 144 73
(a) Rieger A M, Nelson K L, Konowalchuk J D and Barreda D R 2011 Modified annexin V/propidium iodide apoptosis assay for accurate assessment of cell death J. Vis. Exp. 50 2597; (b) Raza MK, Mitra K, Shettar A, Basu U, Kondaiah P and Chakravarty A R 2016 Photoactive platinum(ii) \(\upbeta \)-diketonates as dual action anticancer agents Dalton Trans. 45 13234
Acknowledgements
We thank the National Institute of Technology, Manipur for providing a research facility to carry out the work. We gratefully thank the Board of Research in Nuclear Science (BRNS), Mumbai (37(2)/14/18/2017-BRNS) and Department of Science and Technology (DST) (Women Scientist A Scheme (SR/WOSA/CS-31/2016)), Government of India for providing financial support. We thank Prof. Akhil R. Chakravarty for his help in conducting cytotoxicity studies at IISc Bangalore.
Author information
Authors and Affiliations
Corresponding author
Additional information
Corrected publication 2019.
The original version of this article was revised. Figure 1 was published incorrectly and it is now corrected.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Binita Chanu, S., Raza, M.K., Banerjee, S. et al. ROS dependent antitumour activity of photo-activated iron(III) complexes of amino acids. J Chem Sci 131, 9 (2019). https://doi.org/10.1007/s12039-018-1584-3
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12039-018-1584-3