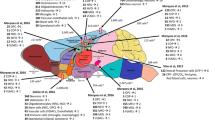
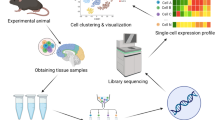
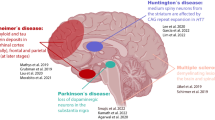
Abstract
Neurological diseases are a major cause of the global burden of disease. Although the mechanisms of the occurrence and development of neurological diseases are not fully clear, most of them are associated with cells mediating neuroinflammation. Yet medications and other therapeutic options to improve treatment are still very limited. Single-cell RNA sequencing (scRNA-seq), as a delightfully potent breakthrough technology, not only identifies various cell types and response states but also uncovers cell-specific gene expression changes, gene regulatory networks, intercellular communication, and cellular movement trajectories, among others, in different cell types. In this review, we describe the technology of scRNA-seq in detail and discuss and summarize the application of scRNA-seq in exploring neurological diseases, elaborating the corresponding specific mechanisms of the diseases as well as providing a reliable basis for new therapeutic approaches. Finally, we affirm that scRNA-seq promotes the development of the neuroscience field and enables us to have a deeper cellular understanding of neurological diseases in the future, which provides strong support for the treatment of neurological diseases and the improvement of patients’ prognosis.





Similar content being viewed by others
Data Availability
Data sharing is not applicable to this article as no datasets were generated or analyzed during the current study.
References
Feigin VL, Vos T, Nichols E, Owolabi MO, Carroll WM, Dichgans M, Deuschl G, Parmar P, Brainin M, Murray C (2020) The global burden of neurological disorders: translating evidence into policy. Lancet Neurol 19(3):255–265. https://doi.org/10.1016/S1474-4422(19)30411-9
Magid-Bernstein J, Girard R, Polster S, Srinath A, Romanos S, Awad IA, Sansing LH (2022) Cerebral hemorrhage: pathophysiology, treatment, and future directions. Circ Res 130(8):1204–1229. https://doi.org/10.1161/CIRCRESAHA.121.319949
Colín-Castelán D, Zaina S (2019) Associations between atherosclerosis and neurological diseases, beyond ischemia-induced cerebral damage. Rev Endocr Metab Disord 20(1):15–25. https://doi.org/10.1007/s11154-019-09486-z
Wang P, Luo M, Zhou W, Jin X, Xu Z, Yan S, Li Y, Xu C, Cheng R, Huang Y, Lin X, Yao L, Nie H, Jiang Q (2022) Global characterization of peripheral B cells in Parkinson’s disease by single-cell RNA and BCR sequencing. Front Immunol 13:814239. https://doi.org/10.3389/fimmu.2022.814239
Wang Y-J, Li Z-X, Gu H-Q, Zhai Y, Zhou Q, Jiang Y, Zhao X-Q, Wang Y-L, Yang X, Wang C-J, Meng X, Li H, Liu L-P, Jing J, Wu J, Xu A-D, Dong Q, Wang D, Wang W-Z, … China Stroke Statistics Writing Committee (2022) China Stroke Statistics: an update on the 2019 report from the National Center for Healthcare Quality Management in Neurological Diseases, China National Clinical Research Center for Neurological Diseases, the Chinese Stroke Association, National Center for Chronic and Non-communicable Disease Control and Prevention, Chinese Center for Disease Control and Prevention and Institute for Global Neuroscience and Stroke Collaborations. Stroke Vasc Neurol 7(5):415–450.https://doi.org/10.1136/svn-2021-001374
Bhat ZF, Morton JD, Mason S, Bekhit AE-DA, Bhat HF (2019) Obesity and neurological disorders: dietary perspective of a global menace. Crit Rev Food Sci Nutr 59(8):1294–1310. https://doi.org/10.1080/10408398.2017.1404442
Kim DW, Tu KJ, Wei A, Lau AJ, Gonzalez-Gil A, Cao T, Braunstein K, Ling JP, Troncoso JC, Wong PC, Blackshaw S, Schnaar RL, Li T (2022) Amyloid-beta and tau pathologies act synergistically to induce novel disease stage-specific microglia subtypes. Mol Neurodegener 17(1):83. https://doi.org/10.1186/s13024-022-00589-x
Kim H, Harrison FE, Aschner M, Bowman AB (2022) Exposing the role of metals in neurological disorders: a focus on manganese. Trends Mol Med 28(7):555–568. https://doi.org/10.1016/j.molmed.2022.04.011
Tsao CW, Aday AW, Almarzooq ZI, Anderson CAM, Arora P, Avery CL, Baker-Smith CM, Beaton AZ, Boehme AK, Buxton AE, Commodore-Mensah Y, Elkind MSV, Evenson KR, Eze-Nliam C, Fugar S, Generoso G, Heard DG, Hiremath S, Ho JE, … American Heart Association Council on Epidemiology and Prevention Statistics Committee and Stroke Statistics Subcommittee (2023) Heart disease and stroke statistics-2023 update: a report from the American Heart Association. Circulation 147(8):e93–e621.https://doi.org/10.1161/CIR.0000000000001123
Coulson RL, Mourrain P, Wang GX (2022) Sleep deficiency as a driver of cellular stress and damage in neurological disorders. Sleep Med Rev 63:101616. https://doi.org/10.1016/j.smrv.2022.101616
Gilhus NE, Deuschl G (2019) Neuroinflammation—a common thread in neurological disorders. Nat Rev Neurol 15(8):429–430. https://doi.org/10.1038/s41582-019-0227-8
Esmaeili Y, Yarjanli Z, Pakniya F, Bidram E, Łos MJ, Eshraghi M, Klionsky DJ, Ghavami S, Zarrabi A (2022) Targeting autophagy, oxidative stress, and ER stress for neurodegenerative disease treatment. J Control Release: Off J Control Release Soc 345:147–175. https://doi.org/10.1016/j.jconrel.2022.03.001
David S, Ryan F, Jhelum P, Kroner A (2023) Ferroptosis in neurological disease. Neuroscientist: Rev J Bringing Neurobiol, Neurol Psychiatry 29(5):591–615. https://doi.org/10.1177/10738584221100183
Falabella M, Vernon HJ, Hanna MG, Claypool SM, Pitceathly RDS (2021) Cardiolipin, mitochondria, and neurological disease. Trends Endocrinol Metab 32(4):224–237. https://doi.org/10.1016/j.tem.2021.01.006
Peng C, Trojanowski JQ, Lee VM-Y (2020) Protein transmission in neurodegenerative disease. Nat Rev Neurol 16(4):199–212. https://doi.org/10.1038/s41582-020-0333-7
Gorji A (2022) Neuroinflammation: the pathogenic mechanism of neurological disorders. Int J Mol Sci 23(10):5744. https://doi.org/10.3390/ijms23105744
Ingelfinger F, Beltrán E, Gerdes LA, Becher B (2022) Single-cell multiomics in neuroinflammation. Curr Opin Immunol 76:102180. https://doi.org/10.1016/j.coi.2022.102180
Tang F, Barbacioru C, Wang Y, Nordman E, Lee C, Xu N, Wang X, Bodeau J, Tuch BB, Siddiqui A, Lao K, Surani MA (2009) MRNA-Seq whole-transcriptome analysis of a single cell. Nat Methods 6(5):377–382. https://doi.org/10.1038/nmeth.1315
Hwang B, Lee JH, Bang D (2018) Single-cell RNA sequencing technologies and bioinformatics pipelines. Exp Mol Med 50(8):1–14. https://doi.org/10.1038/s12276-018-0071-8
Cha J, Lee I (2020) Single-cell network biology for resolving cellular heterogeneity in human diseases. Exp Mol Med 52(11):1798–1808. https://doi.org/10.1038/s12276-020-00528-0
Travaglini KJ, Nabhan AN, Penland L, Sinha R, Gillich A, Sit RV, Chang S, Conley SD, Mori Y, Seita J, Berry GJ, Shrager JB, Metzger RJ, Kuo CS, Neff N, Weissman IL, Quake SR, Krasnow MA (2020) A molecular cell atlas of the human lung from single-cell RNA sequencing. Nature 587(7835):619–625. https://doi.org/10.1038/s41586-020-2922-4
Ou Z, Lin S, Qiu J, Ding W, Ren P, Chen D, Wang J, Tong Y, Wu D, Chen A, Deng Y, Cheng M, Peng T, Lu H, Yang H, Wang J, Jin X, Ma D, Xu X, … Wu P (2022) Single-nucleus RNA sequencing and spatial transcriptomics reveal the immunological microenvironment of cervical squamous cell carcinoma. Adv Sci (Weinheim, Baden-Wurttemberg, Germany) 9(29):e2203040. https://doi.org/10.1002/advs.202203040
Ranzoni AM, Tangherloni A, Berest I, Riva SG, Myers B, Strzelecka PM, Xu J, Panada E, Mohorianu I, Zaugg JB, Cvejic A (2021) Integrative single-cell RNA-Seq and ATAC-Seq analysis of human developmental hematopoiesis. Cell Stem Cell 28(3):472-487.e7. https://doi.org/10.1016/j.stem.2020.11.015
Han J, DePinho RA, Maitra A (2021) Single-cell RNA sequencing in pancreatic cancer. Nat Rev Gastroenterol Hepatol 18(7):451–452. https://doi.org/10.1038/s41575-021-00471-z
Denisenko E, Guo BB, Jones M, Hou R, de Kock L, Lassmann T, Poppe D, Clément O, Simmons RK, Lister R, Forrest ARR (2020) Systematic assessment of tissue dissociation and storage biases in single-cell and single-nucleus RNA-seq workflows. Genome Biol 21(1):130. https://doi.org/10.1186/s13059-020-02048-6
Adam M, Potter AS, Potter SS (2017) Psychrophilic proteases dramatically reduce single-cell RNA-seq artifacts: a molecular atlas of kidney development. Development (Cambridge, England) 144(19):3625–3632. https://doi.org/10.1242/dev.151142
Tavakoli H, Zhou W, Ma L, Perez S, Ibarra A, Xu F, Zhan S, Li X (2019) Recent advances in microfluidic platforms for single-cell analysis in cancer biology, diagnosis and therapy. Trends Anal Chem: TRAC 117:13–26. https://doi.org/10.1016/j.trac.2019.05.010
Prakadan SM, Shalek AK, Weitz DA (2017) Scaling by shrinking: empowering single-cell 《omics》 with microfluidic devices. Nat Rev Genet 18(6):345–361. https://doi.org/10.1038/nrg.2017.15
Mazutis L, Gilbert J, Ung WL, Weitz DA, Griffiths AD, Heyman JA (2013) Single-cell analysis and sorting using droplet-based microfluidics. Nat Protoc 8(5):870–891. https://doi.org/10.1038/nprot.2013.046
Zilionis R, Nainys J, Veres A, Savova V, Zemmour D, Klein AM, Mazutis L (2017) Single-cell barcoding and sequencing using droplet microfluidics. Nat Protoc 12(1):44–73. https://doi.org/10.1038/nprot.2016.154
Islam S, Kjällquist U, Moliner A, Zajac P, Fan J-B, Lönnerberg P, Linnarsson S (2011) Characterization of the single-cell transcriptional landscape by highly multiplex RNA-seq. Genome Res 21(7):1160–1167. https://doi.org/10.1101/gr.110882.110
Luo G, Gao Q, Zhang S, Yan B (2020) Probing infectious disease by single-cell RNA sequencing: progresses and perspectives. Comput Struct Biotechnol J 18:2962–2971. https://doi.org/10.1016/j.csbj.2020.10.016
Ziegenhain C, Vieth B, Parekh S, Reinius B, Guillaumet-Adkins A, Smets M, Leonhardt H, Heyn H, Hellmann I, Enard W (2017) Comparative analysis of single-cell RNA sequencing methods. Mol Cell 65(4):631-643.e4. https://doi.org/10.1016/j.molcel.2017.01.023
Macosko EZ, Basu A, Satija R, Nemesh J, Shekhar K, Goldman M, Tirosh I, Bialas AR, Kamitaki N, Martersteck EM, Trombetta JJ, Weitz DA, Sanes JR, Shalek AK, Regev A, McCarroll SA (2015) Highly parallel genome-wide expression profiling of individual cells using nanoliter droplets. Cell 161(5):1202–1214. https://doi.org/10.1016/j.cell.2015.05.002
Balzer MS, Ma Z, Zhou J, Abedini A, Susztak K (2021) How to get started with single cell RNA sequencing data analysis. J Am Soc Nephrol 32(6):1279–1292. https://doi.org/10.1681/ASN.2020121742
Hong R, Koga Y, Bandyadka S, Leshchyk A, Wang Y, Akavoor V, Cao X, Sarfraz I, Wang Z, Alabdullatif S, Jansen F, Yajima M, Johnson WE, Campbell JD (2022) Comprehensive generation, visualization, and reporting of quality control metrics for single-cell RNA sequencing data. Nat Commun 13(1):1688. https://doi.org/10.1038/s41467-022-29212-9
Haghverdi L, Lun ATL, Morgan MD, Marioni JC (2018) Batch effects in single-cell RNA-sequencing data are corrected by matching mutual nearest neighbors. Nat Biotechnol 36(5):421–427. https://doi.org/10.1038/nbt.4091
Wu Y, Zhang K (2020) Tools for the analysis of high-dimensional single-cell RNA sequencing data. Nat Rev Nephrol 16(7):408–421. https://doi.org/10.1038/s41581-020-0262-0
Ding Q, Liu S, Yao Y, Liu H, Cai T, Han L (2022) Global, regional, and national burden of ischemic stroke, 1990–2019. Neurology 98(3):e279–e290. https://doi.org/10.1212/WNL.0000000000013115
GBD 2019 Stroke Collaborators (2021) Global, regional, and national burden of stroke and its risk factors, 1990–2019: a systematic analysis for the Global Burden of Disease Study 2019. Lancet Neurol 20(10):795–820. https://doi.org/10.1016/S1474-4422(21)00252-0
Tu W-J, Zhao Z, Yin P, Cao L, Zeng J, Chen H, Fan D, Fang Q, Gao P, Gu Y, Tan G, Han J, He L, Hu B, Hua Y, Kang D, Li H, Liu J, Liu Y, … Wang L (2023) Estimated burden of stroke in China in 2020. JAMA Network Open 6(3):e231455. https://doi.org/10.1001/jamanetworkopen.2023.1455
Walter K (2022) What is acute ischemic stroke? JAMA 327(9):885. https://doi.org/10.1001/jama.2022.1420
Dhandapani R, Neri M, Bernhard M, Brzak I, Schweizer T, Rudin S, Joller S, Berth R, Kernen J, Neuhaus A, Waldt A, Cuttat R, Naumann U, Keller CG, Roma G, Feuerbach D, Shimshek DR, Neumann U, Gasparini F, Galimberti I (2022) Sustained Trem2 stabilization accelerates microglia heterogeneity and Aβ pathology in a mouse model of Alzheimer’s disease. Cell Rep 39(9):110883. https://doi.org/10.1016/j.celrep.2022.110883
Richards LG, Cramer SC (2021) Advances in stroke: therapies targeting stroke recovery. Stroke 52(1):348–350. https://doi.org/10.1161/STROKEAHA.120.033231
Montaner J, Ramiro L, Simats A, Tiedt S, Makris K, Jickling GC, Debette S, Sanchez J-C, Bustamante A (2020) Multilevel omics for the discovery of biomarkers and therapeutic targets for stroke. Nat Rev Neurol 16(5):247–264. https://doi.org/10.1038/s41582-020-0350-6
Iadecola C (2017) The neurovascular unit coming of age: a journey through neurovascular coupling in health and disease. Neuron 96(1):17–42. https://doi.org/10.1016/j.neuron.2017.07.030
Ng YS, Bindoff LA, Gorman GS, Klopstock T, Kornblum C, Mancuso M, McFarland R, Sue CM, Suomalainen A, Taylor RW, Thorburn DR, Turnbull DM (2021) Mitochondrial disease in adults: recent advances and future promise. Lancet Neurol 20(7):573–584. https://doi.org/10.1016/S1474-4422(21)00098-3
Guo K, Luo J, Feng D, Wu L, Wang X, Xia L, Tao K, Wu X, Cui W, He Y, Wang B, Zhao Z, Zhang Z (2021) Single-cell RNA sequencing with combined use of bulk RNA sequencing to reveal cell heterogeneity and molecular changes at acute stage of ischemic stroke in mouse cortex penumbra area. Front Cell Dev Biol 9:624711. https://doi.org/10.3389/fcell.2021.624711
Shi L, Sun Z, Su W, Xu F, Xie D, Zhang Q, Dai X, Iyer K, Hitchens TK, Foley LM, Li S, Stolz DB, Chen K, Ding Y, Thomson AW, Leak RK, Chen J, Hu X (2021) Treg cell-derived osteopontin promotes microglia-mediated white matter repair after ischemic stroke. Immunity 54(7):1527-1542.e8. https://doi.org/10.1016/j.immuni.2021.04.022
Zheng D, Liu J, Piao H, Zhu Z, Wei R, Liu K (2022) ROS-triggered endothelial cell death mechanisms: focus on pyroptosis, parthanatos, and ferroptosis. Front Immunol 13:1039241. https://doi.org/10.3389/fimmu.2022.1039241
Zheng K, Lin L, Jiang W, Chen L, Zhang X, Zhang Q, Ren Y, Hao J (2022) Single-cell RNA-seq reveals the transcriptional landscape in ischemic stroke. J Cereb Blood Flow Metab: Off J Int Soc Cereb Blood Flow Metab 42(1):56–73. https://doi.org/10.1177/0271678X211026770
Li X, Lyu J, Li R, Jain V, Shen Y, Del Águila Á, Hoffmann U, Sheng H, Yang W (2022) Single-cell transcriptomic analysis of the immune cell landscape in the aged mouse brain after ischemic stroke. J Neuroinflammation 19(1):83. https://doi.org/10.1186/s12974-022-02447-5
Kim GS, Harmon E, Gutierrez M, Stephenson J, Chauhan A, Banerjee A, Wise Z, Doan A, Wu T, Lee J, Jung JE, McCullough L, Wythe J, Marrelli S (2023) Single-cell analysis identifies Ifi27l2a as a novel gene regulator of microglial inflammation in the context of aging and stroke. Res Square rs.3.rs-2557290. https://doi.org/10.21203/rs.3.rs-2557290/v1
Garcia-Bonilla L, Shahanoor Z, Sciortino R, Nazarzoda O, Racchumi G, Iadecola C, Anrather J (2023) Brain and blood single-cell transcriptomics in acute and subacute phases after experimental stroke. BioRxiv: The Preprint Server for Biology, 2023.03.31.535150. https://doi.org/10.1101/2023.03.31.535150
Zhang Y, Guo Y, Li R, Huang T, Li Y, Xie W, Chen C, Chen W, Wan J, Yu W, Li P (2023) Novel CH25H+ and OASL+ microglia subclusters play distinct roles in cerebral ischemic stroke. J Neuroinflammation 20(1):115. https://doi.org/10.1186/s12974-023-02799-6
Ma H, Li H, Zhang Y, Zhou Y, Liu H, Xu H, Zhu L, Zhang G, Wang J, Li Z, Hong B, Zhou W, Yang P, Liu J (2023) Microglia exhibit distinct heterogeneity rather than M1/M2 polarization within the early stage of acute ischemic stroke. Aging Dis. https://doi.org/10.14336/AD.2023.0505
Liu T, Bai M, Liu M, Li T, Liao Y, Zhao C, Yao M, Wang J, Wen A, Ding Y (2023) Novel synergistic mechanism of 11-keto-β-boswellic acid and Z-Guggulsterone on ischemic stroke revealed by single-cell transcriptomics. Pharmacol Res 193:106803. https://doi.org/10.1016/j.phrs.2023.106803
Jin C, Shi Y, Shi L, Leak RK, Zhang W, Chen K, Ye Q, Hassan S, Lyu J, Hu X, Stetler RA, Bennett MVL, Chen J (2023) Leveraging single-cell RNA sequencing to unravel the impact of aging on stroke recovery mechanisms in mice. Proc Natl Acad Sci USA 120(25):e2300012120. https://doi.org/10.1073/pnas.2300012120
Ma H, Zhou Y, Li Z, Zhu L, Li H, Zhang G, Wang J, Gong H, Xu D, Hua W, Liu P, Zhang X, Zhang Y, Zhang L, Hong B, Zhou W, Yang P, Liu J (2022) Single-cell RNA-sequencing analyses revealed heterogeneity and dynamic changes of metabolic pathways in astrocytes at the acute phase of ischemic stroke. Oxid Med Cell Longev 2022:1817721. https://doi.org/10.1155/2022/1817721
Lin W, Wang Y, Chen Y, Wang Q, Gu Z, Zhu Y (2021) Role of calcium signaling pathway-related gene regulatory networks in ischemic stroke based on multiple WGCNA and single-cell analysis. Oxid Med Cell Longev 2021:8060477. https://doi.org/10.1155/2021/8060477
Zhao N, Qiao W, Li F, Ren Y, Zheng J, Martens YA, Wang X, Li L, Liu C-C, Chen K, Zhu Y, Ikezu TC, Li Z, Meneses AD, Jin Y, Knight JA, Chen Y, Bastea L, Linares C, … Bu G (2022) Elevating microglia TREM2 reduces amyloid seeding and suppresses disease-associated microglia. J Exp Med 219(12):e20212479. https://doi.org/10.1084/jem.20212479
Zhao B, Jiang X (2022) Hsa-miR-518-5p/hsa-miR-3135b regulates the REL/SOD2 pathway in ischemic cerebral infarction. Front Neurol 13:852013. https://doi.org/10.3389/fneur.2022.852013
Chen Z, Wang X, Wu H, Fan Y, Yan Z, Lu C, Ouyang H, Zhang S, Zhang M (2022) X-box binding protein 1 as a key modulator in 《healing endothelial cells》, a novel EC phenotype promoting angiogenesis after MCAO. Cell Mol Biol Lett 27(1):97. https://doi.org/10.1186/s11658-022-00399-5
Xie L, Zhang S, Huang L, Peng Z, Lu H, He Q, Chen R, Hu L, Wang B, Sun B, Yang Q, Xie Q (2023) Single-cell RNA sequencing of peripheral blood reveals that monocytes with high cathepsin S expression aggravate cerebral ischemia-reperfusion injury. Brain Behav Immun 107:330–344. https://doi.org/10.1016/j.bbi.2022.11.001
Cho Y-E, Lee H, Bae HR, Kim H, Yun S, Vorn R, Cashion A, Rucker MJ, Afzal M, Latour L, Gill J (2022) Circulating immune cell landscape in patients who had mild ischaemic stroke. Stroke and Vascular Neurology 7(4):319–327. https://doi.org/10.1136/svn-2021-001224
Frazier AP, Mitchell DN, Given KS, Hunn G, Burch AM, Childs CR, Moreno-Garcia M, Corigilano MR, Quillinan N, Macklin WB, Herson PS, Dingman AL (2023) Chronic changes in oligodendrocyte sub-populations after middle cerebral artery occlusion in neonatal mice. Glia 71(6):1429–1450. https://doi.org/10.1002/glia.24349
Wang R, Li H, Ling C, Zhang X, Lu J, Luan W, Zhang J, Shi L (2023) A novel phenotype of B cells associated with enhanced phagocytic capability and chemotactic function after ischemic stroke. Neural Regen Res 18(11):2413–2423. https://doi.org/10.4103/1673-5374.371365
Cao G-Z, Hou J-Y, Zhou R, Tian L-L, Wang M-L, Zhang Y, Xu H, Yang H-J, Zhang J-J (2023) Single-cell RNA sequencing reveals that VIM and IFITM3 are vital targets of Dengzhan Shengmai capsule to protect against cerebral ischemic injury. J Ethnopharmacol 311:116439. https://doi.org/10.1016/j.jep.2023.116439
Shi X, Luo L, Wang J, Shen H, Li Y, Mamtilahun M, Liu C, Shi R, Lee J-H, Tian H, Zhang Z, Wang Y, Chung W-S, Tang Y, Yang G-Y (2021) Stroke subtype-dependent synapse elimination by reactive gliosis in mice. Nat Commun 12(1):6943. https://doi.org/10.1038/s41467-021-27248-x
Masuda T, Sankowski R, Staszewski O, Prinz M (2020) Microglia heterogeneity in the single-cell era. Cell Rep 30(5):1271–1281. https://doi.org/10.1016/j.celrep.2020.01.010
Candelario-Jalil E, Dijkhuizen RM, Magnus T (2022) Neuroinflammation, stroke, blood-brain barrier dysfunction, and imaging modalities. Stroke 53(5):1473–1486. https://doi.org/10.1161/STROKEAHA.122.036946
Schlomann U, Rathke-Hartlieb S, Yamamoto S, Jockusch H, Bartsch JW (2000) Tumor necrosis factor alpha induces a metalloprotease-disintegrin, ADAM8 (CD 156): Implications for neuron-glia interactions during neurodegeneration. J Neurosci: Off J Soc Neurosci 20(21):7964–7971. https://doi.org/10.1523/JNEUROSCI.20-21-07964.2000
Liu Z, Chopp M (2016) Astrocytes, therapeutic targets for neuroprotection and neurorestoration in ischemic stroke. Prog Neurobiol 144:103–120. https://doi.org/10.1016/j.pneurobio.2015.09.008
Yamagata K (2022) Lactate supply from astrocytes to neurons and its role in ischemic stroke-induced neurodegeneration. Neuroscience 481:219–231. https://doi.org/10.1016/j.neuroscience.2021.11.035
Umebashi K, Tokito A, Yamamoto M, Jougasaki M (2018) Interleukin-33 induces interleukin-8 expression via JNK/c-Jun/AP-1 pathway in human umbilical vein endothelial cells. PLoS One 13(1):e0191659. https://doi.org/10.1371/journal.pone.0191659
Meng H, Song Y, Zhu J, Liu Q, Lu P, Ye N, Zhang Z, Pang Y, Qi J, Wu H (2016) LRG1 promotes angiogenesis through upregulating the TGF-β1 pathway in ischemic rat brain. Mol Med Rep 14(6):5535–5543. https://doi.org/10.3892/mmr.2016.5925
Kim E, Cho S (2016) Microglia and monocyte-derived macrophages in stroke. Neurother: J Am Soc Exp NeuroTher 13(4):702–718. https://doi.org/10.1007/s13311-016-0463-1
Kanazawa M, Ninomiya I, Hatakeyama M, Takahashi T, Shimohata T (2017) Microglia and monocytes/macrophages polarization reveal novel therapeutic mechanism against stroke. Int J Mol Sci 18(10):2135. https://doi.org/10.3390/ijms18102135
De Vlaminck K, Van Hove H, Kancheva D, Scheyltjens I, Pombo Antunes AR, Bastos J, Vara-Perez M, Ali L, Mampay M, Deneyer L, Miranda JF, Cai R, Bouwens L, De Bundel D, Caljon G, Stijlemans B, Massie A, Van Ginderachter JA, Vandenbroucke RE, Movahedi K (2022) Differential plasticity and fate of brain-resident and recruited macrophages during the onset and resolution of neuroinflammation. Immunity 55(11):2085-2102.e9. https://doi.org/10.1016/j.immuni.2022.09.005
Hernández IH, Villa-González M, Martín G, Soto M, Pérez-Álvarez MJ (2021) Glial cells as therapeutic approaches in brain ischemia-reperfusion injury. Cells 10(7):1639. https://doi.org/10.3390/cells10071639
Villa Gonzalez M, Pérez-Álvarez MJ (2021) A 3R-Tau-mediated mechanism in oligodendrocytes: could it be the key for neuroprotection after stroke? Neural Regen Res 16(12):2401–2402. https://doi.org/10.4103/1673-5374.313027
Doyle KP, Quach LN, Solé M, Axtell RC, Nguyen T-VV, Soler-Llavina GJ, Jurado S, Han J, Steinman L, Longo FM, Schneider JA, Malenka RC, Buckwalter MS (2015) B-lymphocyte-mediated delayed cognitive impairment following stroke. J Neurosci: Off J Soc Neurosci 35(5):2133–2145. https://doi.org/10.1523/JNEUROSCI.4098-14.2015
Endres M, Moro MA, Nolte CH, Dames C, Buckwalter MS, Meisel A (2022) Immune pathways in etiology, acute phase, and chronic sequelae of ischemic stroke. Circ Res 130(8):1167–1186. https://doi.org/10.1161/CIRCRESAHA.121.319994
Ye H, Robak LA, Yu M, Cykowski M, Shulman JM (2023) Genetics and pathogenesis of Parkinson’s syndrome. Annu Rev Pathol 18:95–121. https://doi.org/10.1146/annurev-pathmechdis-031521-034145
Ye J, Huang F, Zeng H, Xu X, Wu G, Tian S, Zhao J, Zhang W (2023) Multi-omics and network pharmacology study reveals the effects of Dengzhan Shengmai capsule against neuroinflammatory injury and thrombosis induced by ischemic stroke. J Ethnopharmacol 305:116092. https://doi.org/10.1016/j.jep.2022.116092
Gustavsson A, Norton N, Fast T, Frölich L, Georges J, Holzapfel D, Kirabali T, Krolak-Salmon P, Rossini PM, Ferretti MT, Lanman L, Chadha AS, van der Flier WM (2023) Global estimates on the number of persons across the Alzheimer’s disease continuum. Alzheimers Dement: J Alzheimers Assoc 19(2):658–670. https://doi.org/10.1002/alz.12694
GBD 2016 Dementia Collaborators (2019) Global, regional, and national burden of Alzheimer’s disease and other dementias, 1990–2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet Neurol 18(1):88–106. https://doi.org/10.1016/S1474-4422(18)30403-4
GBD 2019 Diseases and Injuries Collaborators (2020) Global burden of 369 diseases and injuries in 204 countries and territories, 1990–2019: a systematic analysis for the Global Burden of Disease Study 2019. Lancet (London, England) 396(10258):1204–1222. https://doi.org/10.1016/S0140-6736(20)30925-9
Hardy J, Escott-Price V (2019) Genes, pathways and risk prediction in Alzheimer’s disease. Hum Mol Genet 28(R2):R235–R240. https://doi.org/10.1093/hmg/ddz163
Guo T, Zhang D, Zeng Y, Huang TY, Xu H, Zhao Y (2020) Molecular and cellular mechanisms underlying the pathogenesis of Alzheimer’s disease. Mol Neurodegener 15(1):40. https://doi.org/10.1186/s13024-020-00391-7
DeTure MA, Dickson DW (2019) The neuropathological diagnosis of Alzheimer’s disease. Mol Neurodegener 14(1):32. https://doi.org/10.1186/s13024-019-0333-5
Calsolaro V, Edison P (2016) Neuroinflammation in Alzheimer’s disease: current evidence and future directions. Alzheimers Dement: J Alzheimers Assoc 12(6):719–732. https://doi.org/10.1016/j.jalz.2016.02.010
Jorfi M, Maaser-Hecker A, Tanzi RE (2023) The neuroimmune axis of Alzheimer’s disease. Genome Medicine 15(1):6. https://doi.org/10.1186/s13073-023-01155-w
Johnson TS, Xiang S, Helm BR, Abrams ZB, Neidecker P, Machiraju R, Zhang Y, Huang K, Zhang J (2020) Spatial cell type composition in normal and Alzheimer’s human brains is revealed using integrated mouse and human single cell RNA sequencing. Sci Rep 10(1):18014. https://doi.org/10.1038/s41598-020-74917-w
Keren-Shaul H, Spinrad A, Weiner A, Matcovitch-Natan O, Dvir-Szternfeld R, Ulland TK, David E, Baruch K, Lara-Astaiso D, Toth B, Itzkovitz S, Colonna M, Schwartz M, Amit I (2017) A unique microglia type associated with restricting development of Alzheimer’s disease. Cell 169(7):1276-1290.e17. https://doi.org/10.1016/j.cell.2017.05.018
Krasemann S, Madore C, Cialic R, Baufeld C, Calcagno N, El Fatimy R, Beckers L, O’Loughlin E, Xu Y, Fanek Z, Greco DJ, Smith ST, Tweet G, Humulock Z, Zrzavy T, Conde-Sanroman P, Gacias M, Weng Z, Chen H, … Butovsky O (2017) The TREM2-APOE pathway drives the transcriptional phenotype of dysfunctional microglia in neurodegenerative diseases. Immunity 47(3):566–581.e9. https://doi.org/10.1016/j.immuni.2017.08.008
Mathys H, Adaikkan C, Gao F, Young JZ, Manet E, Hemberg M, De Jager PL, Ransohoff RM, Regev A, Tsai L-H (2017) Temporal tracking of microglia activation in neurodegeneration at single-cell resolution. Cell Rep 21(2):366–380. https://doi.org/10.1016/j.celrep.2017.09.039
Lau S-F, Chen C, Fu W-Y, Qu JY, Cheung TH, Fu AKY, Ip NY (2020) IL-33-PU.1 Transcriptome reprogramming drives functional state transition and clearance activity of microglia in Alzheimer’s disease. Cell Rep 31(3):107530. https://doi.org/10.1016/j.celrep.2020.107530
Alsema AM, Jiang Q, Kracht L, Gerrits E, Dubbelaar ML, Miedema A, Brouwer N, Hol EM, Middeldorp J, van Dijk R, Woodbury M, Wachter A, Xi S, Möller T, Biber KP, Kooistra SM, Boddeke EWGM, Eggen BJL (2020) Profiling microglia from Alzheimer’s disease donors and non-demented elderly in acute human postmortem cortical tissue. Front Mol Neurosci 13:134. https://doi.org/10.3389/fnmol.2020.00134
Olah M, Menon V, Habib N, Taga MF, Ma Y, Yung CJ, Cimpean M, Khairallah A, Coronas-Samano G, Sankowski R, Grün D, Kroshilina AA, Dionne D, Sarkis RA, Cosgrove GR, Helgager J, Golden JA, Pennell PB, Prinz M, … De Jager PL (2020) Single cell RNA sequencing of human microglia uncovers a subset associated with Alzheimer’s disease. Nat Commun 11(1):6129. https://doi.org/10.1038/s41467-020-19737-2
Yang HS, Onos KD, Choi K, Keezer KJ, Skelly DA, Carter GW, Howell GR (2021) Natural genetic variation determines microglia heterogeneity in wild-derived mouse models of Alzheimer’s disease. Cell Rep 34(6):108739. https://doi.org/10.1016/j.celrep.2021.108739
Lee S-H, Meilandt WJ, Xie L, Gandham VD, Ngu H, Barck KH, Rezzonico MG, Imperio J, Lalehzadeh G, Huntley MA, Stark KL, Foreman O, Carano RAD, Friedman BA, Sheng M, Easton A, Bohlen CJ, Hansen DV (2021) Trem2 restrains the enhancement of tau accumulation and neurodegeneration by β-amyloid pathology. Neuron 109(8):1283-1301.e6. https://doi.org/10.1016/j.neuron.2021.02.010
Bhattacherjee A, Jung J, Zia S, Ho M, Eskandari-Sedighi G, St Laurent CD, McCord KA, Bains A, Sidhu G, Sarkar S, Plemel JR, Macauley MS (2021) The CD33 short isoform is a gain-of-function variant that enhances Aβ1-42 phagocytosis in microglia. Mol Neurodegener 16(1):19. https://doi.org/10.1186/s13024-021-00443-6
Lodder C, Scheyltjens I, Stancu IC, Botella Lucena P, Gutiérrez de Ravé M, Vanherle S, Vanmierlo T, Cremers N, Vanrusselt H, Brône B, Hanseeuw B, Octave J-N, Bottelbergs A, Movahedi K, Dewachter I (2021) CSF1R inhibition rescues tau pathology and neurodegeneration in an A/T/N model with combined AD pathologies, while preserving plaque associated microglia. Acta Neuropathol Commun 9(1):108. https://doi.org/10.1186/s40478-021-01204-8
Choi H, Choi Y, Lee EJ, Kim H, Lee Y, Kwon S, Hwang DW, Lee DS, Alzheimer’s Disease Neuroimaging Initiative (2021) Hippocampal glucose uptake as a surrogate of metabolic change of microglia in Alzheimer’s disease. J Neuroinflammation 18(1):190. https://doi.org/10.1186/s12974-021-02244-6
Jian C, Wei L, Mo R, Li R, Liang L, Chen L, Zou C, Meng Y, Liu Y, Zou D (2021) Microglia mediate the occurrence and development of Alzheimer’s disease through ligand-receptor axis communication. Front Aging Neurosci 13:731180. https://doi.org/10.3389/fnagi.2021.731180
Karahan H, Smith DC, Kim B, Dabin LC, Al-Amin MM, Wijeratne HRS, Pennington T, Viana di Prisco G, McCord B, Lin PB-C, Li Y, Peng J, Oblak AL, Chu S, Atwood BK, Kim J (2021) Deletion of Abi3 gene locus exacerbates neuropathological features of Alzheimer’s disease in a mouse model of Aβ amyloidosis. Sci Adv 7(45):eabe3954. https://doi.org/10.1126/sciadv.abe3954
Claes C, England WE, Danhash EP, Kiani Shabestari S, Jairaman A, Chadarevian JP, Hasselmann J, Tsai AP, Coburn MA, Sanchez J, Lim TE, Hidalgo JLS, Tu C, Cahalan MD, Lamb BT, Landreth GE, Spitale RC, Blurton-Jones M, Davtyan H (2022) The P522R protective variant of PLCG2 promotes the expression of antigen presentation genes by human microglia in an Alzheimer’s disease mouse model. Alzheimers Dement: J Alzheimers Assoc 18(10):1765–1778. https://doi.org/10.1002/alz.12577
Tsai AP, Dong C, Lin PB-C, Messenger EJ, Casali BT, Moutinho M, Liu Y, Oblak AL, Lamb BT, Landreth GE, Bissel SJ, Nho K (2022) PLCG2 is associated with the inflammatory response and is induced by amyloid plaques in Alzheimer’s disease. Genome Med 14(1):17. https://doi.org/10.1186/s13073-022-01022-0
Karaahmet B, Le L, Mendes MS, Majewska AK, O’Banion MK (2022) Repopulated microglia induce expression of Cxcl13 with differential changes in Tau phosphorylation but do not impact amyloid pathology. J Neuroinflammation 19(1):173. https://doi.org/10.1186/s12974-022-02532-9
Monzón-Sandoval J, Burlacu E, Agarwal D, Handel AE, Wei L, Davis J, Cowley SA, Cader MZ, Webber C (2022) Lipopolysaccharide distinctively alters human microglia transcriptomes to resemble microglia from Alzheimer’s disease mouse models. Dis Models Mech 15(10):dmm049349. https://doi.org/10.1242/dmm.049349
Jo KW, Lee D, Cha DG, Oh E, Choi YH, Kim S, Park ES, Kim JK, Kim K-T (2022) Gossypetin ameliorates 5xFAD spatial learning and memory through enhanced phagocytosis against Aβ. Alzheimers Res Ther 14(1):158. https://doi.org/10.1186/s13195-022-01096-3
Lee S, Devanney NA, Golden LR, Smith CT, Schwartz JL, Walsh AE, Clarke HA, Goulding DS, Allenger EJ, Morillo-Segovia G, Friday CM, Gorman AA, Hawkinson TR, MacLean SM, Williams HC, Sun RC, Morganti JM, Johnson LA (2023) APOE modulates microglial immunometabolism in response to age, amyloid pathology, and inflammatory challenge. Cell Rep 42(3):112196. https://doi.org/10.1016/j.celrep.2023.112196
Yin Z, Herron S, Silveira S, Kleemann K, Gauthier C, Mallah D, Cheng Y, Margeta MA, Pitts KM, Barry J-L, Subramanian A, Shorey H, Brandao W, Durao A, Delpech J-C, Madore C, Jedrychowski M, Ajay AK, Murugaiyan G, … Butovsky O (2023) Identification of a protective microglial state mediated by miR-155 and interferon-γ signaling in a mouse model of Alzheimer’s disease. Nat Neurosci 26(7):1196–1207. https://doi.org/10.1038/s41593-023-01355-y
van Lengerich B, Zhan L, Xia D, Chan D, Joy D, Park JI, Tatarakis D, Calvert M, Hummel S, Lianoglou S, Pizzo ME, Prorok R, Thomsen E, Bartos LM, Beumers P, Capell A, Davis SS, de Weerd L, Dugas JC, … Monroe KM (2023) A TREM2-activating antibody with a blood-brain barrier transport vehicle enhances microglial metabolism in Alzheimer’s disease models. Nat Neurosci 26(3):416–429. https://doi.org/10.1038/s41593-022-01240-0
De Schepper S, Ge JZ, Crowley G, Ferreira LSS, Garceau D, Toomey CE, Sokolova D, Rueda-Carrasco J, Shin S-H, Kim J-S, Childs T, Lashley T, Burden JJ, Sasner M, Sala Frigerio C, Jung S, Hong S (2023) Perivascular cells induce microglial phagocytic states and synaptic engulfment via SPP1 in mouse models of Alzheimer’s disease. Nat Neurosci 26(3):406–415. https://doi.org/10.1038/s41593-023-01257-z
Pérez MJ, Ivanyuk D, Panagiotakopoulou V, Di Napoli G, Kalb S, Brunetti D, Al-Shaana R, Kaeser SA, Fraschka SA-K, Jucker M, Zeviani M, Viscomi C, Deleidi M (2021) Loss of function of the mitochondrial peptidase PITRM1 induces proteotoxic stress and Alzheimer’s disease-like pathology in human cerebral organoids. Mol Psychiatry 26(10):5733–5750. https://doi.org/10.1038/s41380-020-0807-4
Farmer BC, Williams HC, Devanney NA, Piron MA, Nation GK, Carter DJ, Walsh AE, Khanal R, Young LEA, Kluemper JC, Hernandez G, Allenger EJ, Mooney R, Golden LR, Smith CT, Brandon JA, Gupta VA, Kern PA, Gentry MS, … Johnson LA (2021) APOΕ4 lowers energy expenditure in females and impairs glucose oxidation by increasing flux through aerobic glycolysis. Mol Neurodegener 16(1):62. https://doi.org/10.1186/s13024-021-00483-y
Dang Y, He Q, Yang S, Sun H, Liu Y, Li W, Tang Y, Zheng Y, Wu T (2022) FTH1- and SAT1-induced astrocytic ferroptosis is involved in Alzheimer’s disease: evidence from single-cell transcriptomic analysis. Pharmaceuticals (Basel, Switzerland) 15(10):1177. https://doi.org/10.3390/ph15101177
Soreq L, Bird H, Mohamed W, Hardy J (2023) Single-cell RNA sequencing analysis of human Alzheimer’s disease brain samples reveals neuronal and glial specific cells differential expression. PLoS One 18(2):e0277630. https://doi.org/10.1371/journal.pone.0277630
Sun Y, Zhang H, Zhang X, Wang W, Chen Y, Cai Z, Wang Q, Wang J, Shi Y (2023) Promotion of astrocyte-neuron glutamate-glutamine shuttle by SCFA contributes to the alleviation of Alzheimer’s disease. Redox Biol 62:102690. https://doi.org/10.1016/j.redox.2023.102690
Gate D, Saligrama N, Leventhal O, Yang AC, Unger MS, Middeldorp J, Chen K, Lehallier B, Channappa D, De Los Santos MB, McBride A, Pluvinage J, Elahi F, Tam GK-Y, Kim Y, Greicius M, Wagner AD, Aigner L, Galasko DR, … Wyss-Coray T (2020) Clonally expanded CD8 T cells patrol the cerebrospinal fluid in Alzheimer’s disease. Nature 577(7790):399–404. https://doi.org/10.1038/s41586-019-1895-7
Xiong L-L, Xue L-L, Du R-L, Niu R-Z, Chen L, Chen J, Hu Q, Tan Y-X, Shang H-F, Liu J, Yu C-Y, Wang T-H (2021) Single-cell RNA sequencing reveals B cell-related molecular biomarkers for Alzheimer’s disease. Exp Mol Med 53(12):1888–1901. https://doi.org/10.1038/s12276-021-00714-8
Feng W, Zhang Y, Ding S, Chen S, Wang T, Wang Z, Zou Y, Sheng C, Chen Y, Pang Y, Marshall C, Shi J, Nedergaard M, Li Q, Xiao M (2023) B lymphocytes ameliorate Alzheimer’s disease-like neuropathology via interleukin-35. Brain Behav Immun 108:16–31. https://doi.org/10.1016/j.bbi.2022.11.012
Park H, Cho B, Kim H, Saito T, Saido TC, Won K-J, Kim J (2023) Single-cell RNA-sequencing identifies disease-associated oligodendrocytes in male APP NL-G-F and 5XFAD mice. Nat Commun 14(1):802. https://doi.org/10.1038/s41467-023-36519-8
Qi C, Liu F, Zhang W, Han Y, Zhang N, Liu Q, Li H (2022) Alzheimer’s disease alters the transcriptomic profile of natural killer cells at single-cell resolution. Front Immunol 13:1004885. https://doi.org/10.3389/fimmu.2022.1004885
Sarlus H, Heneka MT (2017) Microglia in Alzheimer’s disease. J Clin Investig 127(9):3240–3249. https://doi.org/10.1172/JCI90606
Hansen DV, Hanson JE, Sheng M (2018) Microglia in Alzheimer’s disease. J Cell Biol 217(2):459–472. https://doi.org/10.1083/jcb.201709069
Shi Y, Holtzman DM (2018) Interplay between innate immunity and Alzheimer disease: APOE and TREM2 in the spotlight. Nat Rev Immunol 18(12):759–772. https://doi.org/10.1038/s41577-018-0051-1
Nguyen AT, Wang K, Hu G, Wang X, Miao Z, Azevedo JA, Suh E, Van Deerlin VM, Choi D, Roeder K, Li M, Lee EB (2020) APOE and TREM2 regulate amyloid-responsive microglia in Alzheimer’s disease. Acta Neuropathol 140(4):477–493. https://doi.org/10.1007/s00401-020-02200-3
Ulland TK, Song WM, Huang SC-C, Ulrich JD, Sergushichev A, Beatty WL, Loboda AA, Zhou Y, Cairns NJ, Kambal A, Loginicheva E, Gilfillan S, Cella M, Virgin HW, Unanue ER, Wang Y, Artyomov MN, Holtzman DM, Colonna M (2017) TREM2 maintains microglial metabolic fitness in Alzheimer’s disease. Cell 170(4):649-663.e13. https://doi.org/10.1016/j.cell.2017.07.023
Eskandari-Sedighi G, Jung J, Macauley MS (2023) CD33 isoforms in microglia and Alzheimer’s disease: friend and foe. Mol Aspects Med 90:101111. https://doi.org/10.1016/j.mam.2022.101111
Sims R, van der Lee SJ, Naj AC, Bellenguez C, Badarinarayan N, Jakobsdottir J, Kunkle BW, Boland A, Raybould R, Bis JC, Martin ER, Grenier-Boley B, Heilmann-Heimbach S, Chouraki V, Kuzma AB, Sleegers K, Vronskaya M, Ruiz A, Graham RR, … Schellenberg GD (2017) Rare coding variants in PLCG2, ABI3, and TREM2 implicate microglial-mediated innate immunity in Alzheimer’s disease. Nat Genet 49(9):1373–1384. https://doi.org/10.1038/ng.3916
Andreone BJ, Przybyla L, Llapashtica C, Rana A, Davis SS, van Lengerich B, Lin K, Shi J, Mei Y, Astarita G, Di Paolo G, Sandmann T, Monroe KM, Lewcock JW (2020) Alzheimer’s-associated PLCγ2 is a signaling node required for both TREM2 function and the inflammatory response in human microglia. Nat Neurosci 23(8):927–938. https://doi.org/10.1038/s41593-020-0650-6
Kleineidam L, Chouraki V, Próchnicki T, van der Lee SJ, Madrid-Márquez L, Wagner-Thelen H, Karaca I, Weinhold L, Wolfsgruber S, Boland A, Martino Adami PV, Lewczuk P, Popp J, Brosseron F, Jansen IE, Hulsman M, Kornhuber J, Peters O, Berr C, … Ramirez A (2020) PLCG2 protective variant p.P522R modulates tau pathology and disease progression in patients with mild cognitive impairment. Acta Neuropathol 139(6):1025–1044. https://doi.org/10.1007/s00401-020-02138-6
Carter SF, Herholz K, Rosa-Neto P, Pellerin L, Nordberg A, Zimmer ER (2019) Astrocyte biomarkers in Alzheimer’s disease. Trends Mol Med 25(2):77–95. https://doi.org/10.1016/j.molmed.2018.11.006
Brunetti D, Catania A, Viscomi C, Deleidi M, Bindoff LA, Ghezzi D, Zeviani M (2021) Role of PITRM1 in mitochondrial dysfunction and neurodegeneration. Biomedicines 9(7):833. https://doi.org/10.3390/biomedicines9070833
Park MW, Cha HW, Kim J, Kim JH, Yang H, Yoon S, Boonpraman N, Yi SS, Yoo ID, Moon J-S (2021) NOX4 promotes ferroptosis of astrocytes by oxidative stress-induced lipid peroxidation via the impairment of mitochondrial metabolism in Alzheimer’s diseases. Redox Biol 41:101947. https://doi.org/10.1016/j.redox.2021.101947
Kim K, Wang X, Ragonnaud E, Bodogai M, Illouz T, DeLuca M, McDevitt RA, Gusev F, Okun E, Rogaev E, Biragyn A (2021) Therapeutic B-cell depletion reverses progression of Alzheimer’s disease. Nat Commun 12(1):2185. https://doi.org/10.1038/s41467-021-22479-4
McManus RM, Heneka MT (2020) T cells in Alzheimer’s disease: space invaders. The Lancet Neurology 19(4):285–287. https://doi.org/10.1016/S1474-4422(20)30076-4
Zhang Y, Fung ITH, Sankar P, Chen X, Robison LS, Ye L, D’Souza SS, Salinero AE, Kuentzel ML, Chittur SV, Zhang W, Zuloaga KL, Yang Q (2020) Depletion of NK cells improves cognitive function in the Alzheimer disease mouse model. J Immunol (Baltimore, Md: 1950) 205(2):502–510. https://doi.org/10.4049/jimmunol.2000037
Tolosa E, Garrido A, Scholz SW, Poewe W (2021) Challenges in the diagnosis of Parkinson’s disease. Lancet Neurol 20(5):385–397. https://doi.org/10.1016/S1474-4422(21)00030-2
Ascherio A, Schwarzschild MA (2016) The epidemiology of Parkinson’s disease: risk factors and prevention. Lancet Neurol 15(12):1257–1272. https://doi.org/10.1016/S1474-4422(16)30230-7
Bloem BR, Okun MS, Klein C (2021) Parkinson’s disease. Lancet (London, England) 397(10291):2284–2303. https://doi.org/10.1016/S0140-6736(21)00218-X
Jankovic J, Tan EK (2020) Parkinson’s disease: Etiopathogenesis and treatment. J Neurol Neurosurg Psychiatry 91(8):795–808. https://doi.org/10.1136/jnnp-2019-322338
Doi D, Magotani H, Kikuchi T, Ikeda M, Hiramatsu S, Yoshida K, Amano N, Nomura M, Umekage M, Morizane A, Takahashi J (2020) Pre-clinical study of induced pluripotent stem cell-derived dopaminergic progenitor cells for Parkinson’s disease. Nat Commun 11(1):3369. https://doi.org/10.1038/s41467-020-17165-w
Kee N, Volakakis N, Kirkeby A, Dahl L, Storvall H, Nolbrant S, Lahti L, Björklund ÅK, Gillberg L, Joodmardi E, Sandberg R, Parmar M, Perlmann T (2017) Single-cell analysis reveals a close relationship between differentiating dopamine and subthalamic nucleus neuronal lineages. Cell Stem Cell 20(1):29–40. https://doi.org/10.1016/j.stem.2016.10.003
Hook PW, McClymont SA, Cannon GH, Law WD, Morton AJ, Goff LA, McCallion AS (2018) Single-cell RNA-Seq of mouse dopaminergic neurons informs candidate gene selection for sporadic Parkinson disease. Am J Hum Genet 102(3):427–446. https://doi.org/10.1016/j.ajhg.2018.02.001
Kramer DJ, Risso D, Kosillo P, Ngai J, Bateup HS (2018) Combinatorial expression of Grp and Neurod6 defines dopamine neuron populations with distinct projection patterns and disease vulnerability. ENeuro 5(3):ENEURO.0152–18.2018. https://doi.org/10.1523/ENEURO.0152-18.2018
Lang C, Campbell KR, Ryan BJ, Carling P, Attar M, Vowles J, Perestenko OV, Bowden R, Baig F, Kasten M, Hu MT, Cowley SA, Webber C, Wade-Martins R (2019) Single-cell sequencing of iPSC-dopamine neurons reconstructs disease progression and identifies HDAC4 as a regulator of Parkinson cell phenotypes. Cell Stem Cell 24(1):93-106.e6. https://doi.org/10.1016/j.stem.2018.10.023
Tiklová K, Björklund ÅK, Lahti L, Fiorenzano A, Nolbrant S, Gillberg L, Volakakis N, Yokota C, Hilscher MM, Hauling T, Holmström F, Joodmardi E, Nilsson M, Parmar M, Perlmann T (2019) Single-cell RNA sequencing reveals midbrain dopamine neuron diversity emerging during mouse brain development. Nat Commun 10(1):581. https://doi.org/10.1038/s41467-019-08453-1
Fernandes HJR, Patikas N, Foskolou S, Field SF, Park J-E, Byrne ML, Bassett AR, Metzakopian E (2020) Single-cell transcriptomics of Parkinson’s disease human in vitro models reveals dopamine neuron-specific stress responses. Cell Rep 33(2):108263. https://doi.org/10.1016/j.celrep.2020.108263
Walter J, Bolognin S, Poovathingal SK, Magni S, Gérard D, Antony PMA, Nickels SL, Salamanca L, Berger E, Smits LM, Grzyb K, Perfeito R, Hoel F, Qing X, Ohnmacht J, Bertacchi M, Jarazo J, Ignac T, Monzel AS, … Schwamborn JC (2021) The Parkinson’s-disease-associated mutation LRRK2-G2019S alters dopaminergic differentiation dynamics via NR2F1. Cell Rep 37(3):109864. https://doi.org/10.1016/j.celrep.2021.109864
Liang L, Tian Y, Feng L, Wang C, Feng G, Stacey GN, Shyh-Chang N, Wu J, Hu B, Li W, Hao J, Wang L, Wang Y (2022) Single-cell transcriptomics reveals the cell fate transitions of human dopaminergic progenitors derived from hESCs. Stem Cell Res Ther 13(1):412. https://doi.org/10.1186/s13287-022-03104-7
Birtele M, Storm P, Sharma Y, Kajtez J, Wahlestedt JN, Sozzi E, Nilsson F, Stott S, He XL, Mattsson B, Ottosson DR, Barker RA, Fiorenzano A, Parmar M (2022) Single-cell transcriptional and functional analysis of dopaminergic neurons in organoid-like cultures derived from human fetal midbrain. Development (Cambridge, England) 149(23):dev200504. https://doi.org/10.1242/dev.200504
Kim J, Daadi MM (2019) Non-cell autonomous mechanism of Parkinson’s disease pathology caused by G2019S LRRK2 mutation in Ashkenazi Jewish patient: single cell analysis. Brain Res 1722:146342. https://doi.org/10.1016/j.brainres.2019.146342
Tiklová K, Nolbrant S, Fiorenzano A, Björklund ÅK, Sharma Y, Heuer A, Gillberg L, Hoban DB, Cardoso T, Adler AF, Birtele M, Lundén-Miguel H, Volakakis N, Kirkeby A, Perlmann T, Parmar M (2020) Single cell transcriptomics identifies stem cell-derived graft composition in a model of Parkinson’s disease. Nat Commun 11(1):2434. https://doi.org/10.1038/s41467-020-16225-5
Nilsson F, Storm P, Sozzi E, Hidalgo Gil D, Birtele M, Sharma Y, Parmar M, Fiorenzano A (2021) Single-cell profiling of coding and noncoding genes in human dopamine neuron differentiation. Cells 10(1):137. https://doi.org/10.3390/cells10010137
Uriarte Huarte O, Kyriakis D, Heurtaux T, Pires-Afonso Y, Grzyb K, Halder R, Buttini M, Skupin A, Mittelbronn M, Michelucci A (2021) Single-cell transcriptomics and in situ morphological analyses reveal microglia heterogeneity across the nigrostriatal pathway. Front Immunol 12:639613. https://doi.org/10.3389/fimmu.2021.639613
Li W, Shen J, Wu H, Lin L, Liu Y, Pei Z, Liu G (2023) Transcriptome analysis reveals a two-gene signature links to motor progression and alterations of immune cells in Parkinson’s disease. J Park Dis 13(1):25–38. https://doi.org/10.3233/JPD-223454
Yan S, Si Y, Zhou W, Cheng R, Wang P, Wang D, Ding W, Shi W, Jiang Q, Yang F, Yao L (2023) Single-cell transcriptomics reveals the interaction between peripheral CD4+ CTLs and mesencephalic endothelial cells mediated by IFNG in Parkinson’s disease. Comput Biol Med 158:106801. https://doi.org/10.1016/j.compbiomed.2023.106801
Guan Q, Liu W, Mu K, Hu Q, Xie J, Cheng L, Wang X (2022) Single-cell RNA sequencing of CSF reveals neuroprotective RAC1+ NK cells in Parkinson’s disease. Front Immunol 13:992505. https://doi.org/10.3389/fimmu.2022.992505
Schonhoff AM, Figge DA, Williams GP, Jurkuvenaite A, Gallups NJ, Childers GM, Webster JM, Standaert DG, Goldman JE, Harms AS (2023) Border-associated macrophages mediate the neuroinflammatory response in an alpha-synuclein model of Parkinson disease. Nat Commun 14(1):3754. https://doi.org/10.1038/s41467-023-39060-w
Huang J, Liu L, Qin L, Huang H, Li X (2022) Single-cell transcriptomics uncovers cellular heterogeneity, mechanisms, and therapeutic targets for Parkinson’s disease. Front Genet 13:686739. https://doi.org/10.3389/fgene.2022.686739
Guo J-D, Zhao X, Li Y, Li G-R, Liu X-L (2018) Damage to dopaminergic neurons by oxidative stress in Parkinson’s disease (Review). Int J Mol Med 41(4):1817–1825. https://doi.org/10.3892/ijmm.2018.3406
Pang SY-Y, Lo RCN, Ho PW-L, Liu H-F, Chang EES, Leung C-T, Malki Y, Choi ZY-K, Wong WY, Kung MH-W, Ramsden DB, Ho S-L (2022) LRRK2, GBA and their interaction in the regulation of autophagy: implications on therapeutics in Parkinson’s disease. Transl Neurodegener 11(1):5. https://doi.org/10.1186/s40035-022-00281-6
George S, Rey NL, Tyson T, Esquibel C, Meyerdirk L, Schulz E, Pierce S, Burmeister AR, Madaj Z, Steiner JA, Escobar Galvis ML, Brundin L, Brundin P (2019) Microglia affect α-synuclein cell-to-cell transfer in a mouse model of Parkinson’s disease. Mol Neurodegener 14(1):34. https://doi.org/10.1186/s13024-019-0335-3
Sabatino JJ, Pröbstel A-K, Zamvil SS (2019) B cells in autoimmune and neurodegenerative central nervous system diseases. Nat Rev Neurosci 20(12):728–745. https://doi.org/10.1038/s41583-019-0233-2
Scott KM (2022) B lymphocytes in Parkinson’s disease. J Park Dis 12(s1):S75–S81. https://doi.org/10.3233/JPD-223418
Contaldi E, Magistrelli L, Comi C (2022) T lymphocytes in Parkinson’s disease. J Park Dis 12(s1):S65–S74. https://doi.org/10.3233/JPD-223152
Earls RH, Lee J-K (2020) The role of natural killer cells in Parkinson’s disease. Exp Mol Med 52(9):1517–1525. https://doi.org/10.1038/s12276-020-00505-7
Frosch M, Amann L, Prinz M (2023) CNS-associated macrophages shape the inflammatory response in a mouse model of Parkinson’s disease. Nat Commun 14(1):3753. https://doi.org/10.1038/s41467-023-39061-9
Kim H, Shin J-Y, Lee Y-S, Yun SP, Maeng H-J, Lee Y (2020) Brain endothelial P-glycoprotein level is reduced in Parkinson’s disease via a vitamin D receptor-dependent pathway. Int J Mol Sci 21(22):8538. https://doi.org/10.3390/ijms21228538
Potter SS (2018) Single-cell RNA sequencing for the study of development, physiology and disease. Nat Rev Nephrol 14(8):479–492. https://doi.org/10.1038/s41581-018-0021-7
Ding S, Chen X, Shen K (2020) Single-cell RNA sequencing in breast cancer: understanding tumor heterogeneity and paving roads to individualized therapy. Cancer Commun (London, England) 40(8):329–344. https://doi.org/10.1002/cac2.12078
Han Y, Wang D, Peng L, Huang T, He X, Wang J, Ou C (2022) Single-cell sequencing: a promising approach for uncovering the mechanisms of tumor metastasis. J Hematol Oncol 15(1):59. https://doi.org/10.1186/s13045-022-01280-w
Acknowledgements
We appreciate the figure generated with the help of BioRender (https://app.biorender.com).
Funding
This study is supported by grants from Zhejiang Provincial Medical and Health Technology Project for Young Backbone Talents (Grant 2019RC234), Hangzhou Medical and Health Technology Project (2017Z03) and Hangzhou Science and Technology Development Project (20150733Q11). Above Projects are all from corresponding author, Hao Zhang.
Author information
Authors and Affiliations
Contributions
Conceptualization: Bingjie Yang and Shuqi Hu; writing—original draft preparation: Bingjie Yang; writing—review and editing: Hao Zhang, Bingjie Yang, Shuqi Hu, Yiru Jiang, Lei Xu, Song Shu; supervision: Hao Zhang. All authors have read and agreed to the published version of the manuscript.
Corresponding author
Ethics declarations
Competing Interests
The authors declare no competing interests.
Ethics Approval
Not applicable.
Consent to Participate
Not applicable.
Consent for Publication
All authors read and approved the final manuscript.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Bingjie Yang and Shuqi Hu contributed equally to this work and share the first authorship.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Yang, B., Hu, S., Jiang, Y. et al. Advancements in Single-Cell RNA Sequencing Research for Neurological Diseases. Mol Neurobiol (2024). https://doi.org/10.1007/s12035-024-04126-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12035-024-04126-3