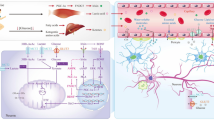
Abstract
Lactate has a novel function different from previously known functions despite its traditional association with hypoxia in skeletal muscle. It plays various direct and indirect physiological functions. It is a vital energy source within the central nervous system (CNS) and a signal transmitter regulating crucial processes, such as angiogenesis and inflammation. Activating lactate and its associated receptors elicits effects like synaptic plasticity and angiogenesis alterations. These effects can significantly influence the astrocyte-neuron lactate shuttle, potentially impacting cognitive performance. Decreased cognitive function relates to different neurodegenerative conditions, including Alzheimer’s disease (AD), ischemic brain injury, and frontotemporal dementia. Therefore, lactic acid has significant potential for treating neurodegenerative disorders. Exercise is a method that induces the production of lactic acid, which is similar to the effect of lactate injections. It is a harmless and natural way to achieve comparable results. Animal experiments demonstrate that high-intensity intermittent exercise can increase vascular endothelial growth factor (VEGF) levels, thus promoting angiogenesis. In vivo, lactate receptor-hydroxycarboxylic acid receptor 1 (HCAR1) activation can occur by various stimuli, including variations in ion concentrations, cyclic adenosine monophosphate (cAMP) level elevations, and fluctuations in the availability of energy substrates. While several articles have been published on the benefits of physical activity on developing Alzheimer’s disease in the CNS, could lactic acid act as a bridge? Understanding how HCAR1 responds to these signals and initiates associated pathways remains incomplete. This review comprehensively analyzes lactate-induced signaling pathways, investigating their influence on neuroinflammation, neurodegeneration, and cognitive decline. Consequently, this study describes the unique role of lactate in the progression of Alzheimer’s disease.


Similar content being viewed by others
Data Availability
Data sharing is not applicable to this article.
References
Breijyeh Z, Karaman R (2020) Comprehensive review on Alzheimer’s disease: causes and treatment. Molecules 25(24). https://doi.org/10.3390/molecules25245789
(2023) 2023 Alzheimer’s disease facts and figures. Alzheimers Dement 19(4):1598-1695. https://doi.org/10.1002/alz.13016
Agüero P, Sainz MJ, Téllez R, Lorda I, Ávila A, García-Ribas G, Rodríguez PP, Gómez-Tortosa E (2021) De novo PS1 mutation (Pro436Gln) in a very early-onset posterior variant of Alzheimer’s disease associated with spasticity: a case report. J Alzheimers Dis 83(3):1011–1016. https://doi.org/10.3233/jad-210420
Beeri MS, Leugrans SE, Delbono O, Bennett DA, Buchman AS (2021) Sarcopenia is associated with incident Alzheimer’s dementia, mild cognitive impairment, and cognitive decline. J Am Geriatr Soc 69(7):1826–1835. https://doi.org/10.1111/jgs.17206
Boyle PA, Buchman AS, Wilson RS, Leurgans SE, Bennett DA (2009) Association of muscle strength with the risk of Alzheimer disease and the rate of cognitive decline in community-dwelling older persons. Arch Neurol 66(11):1339–1344. https://doi.org/10.1001/archneurol.2009.240
Pereira TMC, Côco LZ, Ton AMM, Meyrelles SS, Campos-Toimil M, Campagnaro BP, Vasquez EC (2021) The emerging scenario of the gut-brain axis: the therapeutic actions of the new actor kefir against neurodegenerative diseases. Antioxidants (Basel) 10(11).https://doi.org/10.3390/antiox10111845.
Szegeczki V, Horváth G, Perényi H, Tamás A, Radák Z, Ábrahám D, Zákány R, Reglodi D, et al. (2020) Alzheimer’s disease mouse as a model of testis degeneration. Int J Mol Sci 21(16). https://doi.org/10.3390/ijms21165726
Cortes-Canteli M, Iadecola C (2020) Alzheimer’s disease and vascular aging: JACC focus seminar. J Am Coll Cardiol 75(8):942–951. https://doi.org/10.1016/j.jacc.2019.10.062
Sanna GD, Nusdeo G, Piras MR, Forteleoni A, Murru MR, Saba PS, Dore S, Sotgiu G et al (2019) Cardiac abnormalities in Alzheimer disease: clinical relevance beyond pathophysiological rationale and instrumental findings? JACC Heart Fail 7(2):121–128. https://doi.org/10.1016/j.jchf.2018.10.022
Kaur H, Seeger D, Golovko S, Golovko M, Combs CK (2021) Liver bile acid changes in mouse models of Alzheimer’s disease. Int J Mol Sci 22(14). https://doi.org/10.3390/ijms22147451
Kompanje EJ, Jansen TC, van der Hoven B, Bakker J (2007) The first demonstration of lactic acid in human blood in shock by Johann Joseph Scherer (1814–1869) in January 1843. Intensive Care Med 33(11):1967–1971. https://doi.org/10.1007/s00134-007-0788-7
Haas R, Cucchi D, Smith J, Pucino V, Macdougall CE, Mauro C (2016) Intermediates of metabolism: from bystanders to signalling molecules. Trends Biochem Sci 41(5):460–471. https://doi.org/10.1016/j.tibs.2016.02.003
Liu C, Kuei C, Zhu J, Yu J, Zhang L, Shih A, Mirzadegan T, Shelton J et al (2012) 3,5-Dihydroxybenzoic acid, a specific agonist for hydroxycarboxylic acid 1, inhibits lipolysis in adipocytes. J Pharmacol Exp Ther 341(3):794–801. https://doi.org/10.1124/jpet.112.192799
Sun S, Li H, Chen J, Qian Q (2017) Lactic acid: no longer an inert and end-product of glycolysis. Physiology (Bethesda) 32(6):453–463. https://doi.org/10.1152/physiol.00016.2017
Hadzic A, Nguyen TD, Hosoyamada M, Tomioka NH, Bergersen LH, Storm-Mathisen J, Morland C (2020) The lactate receptor HCA(1) is present in the choroid plexus, the tela choroidea, and the neuroepithelial lining of the dorsal part of the third ventricle. Int J Mol Sci 21(18). https://doi.org/10.3390/ijms21186457
Kuei C, Yu J, Zhu J, Wu J, Zhang L, Shih A, Mirzadegan T, Lovenberg T et al (2011) Study of GPR81, the lactate receptor, from distant species identifies residues and motifs critical for GPR81 functions. Mol Pharmacol 80(5):848–858. https://doi.org/10.1124/mol.111.074500
Ge H, Weiszmann J, Reagan JD, Gupte J, Baribault H, Gyuris T, Chen JL, Tian H et al (2008) Elucidation of signaling and functional activities of an orphan GPCR, GPR81. J Lipid Res 49(4):797–803. https://doi.org/10.1194/jlr.M700513-JLR200
Brown TP, Ganapathy V (2020) Lactate/GPR81 signaling and proton motive force in cancer: role in angiogenesis, immune escape, nutrition, and Warburg phenomenon. Pharmacol Ther 206:107451. https://doi.org/10.1016/j.pharmthera.2019.107451
Morland C, Andersson KA, Haugen ØP, Hadzic A, Kleppa L, Gille A, Rinholm JE, Palibrk V, et al (2017) Exercise induces cerebral VEGF and angiogenesis via the lactate receptor HCAR1. Nat Commun 8:15557. https://doi.org/10.1038/ncomms15557
Alberini CM, Cruz E, Descalzi G, Bessières B, Gao V (2018) Astrocyte glycogen and lactate: new insights into learning and memory mechanisms. Glia 66(6):1244–1262. https://doi.org/10.1002/glia.23250
López-Ortiz S, Valenzuela PL, Seisdedos MM, Morales JS, Vega T, Castillo-García A, Nisticò R, Mercuri NB et al (2021) Exercise interventions in Alzheimer’s disease: a systematic review and meta-analysis of randomized controlled trials. Ageing Res Rev 72:101479. https://doi.org/10.1016/j.arr.2021.101479
Ye L, Jiang Y, Zhang M (2022) Crosstalk between glucose metabolism, lactate production and immune response modulation. Cytokine Growth Factor Rev 68:81–92. https://doi.org/10.1016/j.cytogfr.2022.11.001
Baker J, Brown E, Hill G, Phillips G, Williams R, Davies B (2002) Handgrip contribution to lactate production and leg power during high-intensity exercise. Med Sci Sports Exerc 34(6):1037–1040. https://doi.org/10.1097/00005768-200206000-00021
Vernon C, Letourneau JL (2010) Lactic acidosis: recognition, kinetics, and associated prognosis. Crit Care Clin 26(2):255–283. https://doi.org/10.1016/j.ccc.2009.12.007
Jorfeldt L, Juhlin-Dannfelt A, Karlsson J (1978) Lactate release in relation to tissue lactate in human skeletal muscle during exercise. J Appl Physiol Respir Environ Exerc Physiol 44(3):350–352. https://doi.org/10.1152/jappl.1978.44.3.350
Shulman RG (2005) Glycogen turnover forms lactate during exercise. Exerc Sport Sci Rev 33(4):157–162. https://doi.org/10.1097/00003677-200510000-00002
Richter EA, Kiens B, Saltin B, Christensen NJ, Savard G (1988) Skeletal muscle glucose uptake during dynamic exercise in humans: role of muscle mass. Am J Physiol 254(5 Pt 1):E555-561. https://doi.org/10.1152/ajpendo.1988.254.5.E555
Price TB, Taylor R, Mason GF, Rothman DL, Shulman GI, Shulman RG (1994) Turnover of human muscle glycogen with low-intensity exercise. Med Sci Sports Exerc 26(8):983–991
Brooks GA (2020) Lactate as a fulcrum of metabolism. Redox Biol 35:101454. https://doi.org/10.1016/j.redox.2020.101454
Baik SH, Kang S, Lee W, Choi H, Chung S, Kim JI, Mook-Jung I (2019) A breakdown in metabolic reprogramming causes microglia dysfunction in Alzheimer’s disease. Cell Metab 30(3):493-507.e496. https://doi.org/10.1016/j.cmet.2019.06.005
Solas M, Zamarbide M, Ardanaz CG, Ramírez MJ, Pérez-Mediavilla A (2022) The cognitive improvement and alleviation of brain hypermetabolism caused by FFAR3 ablation in Tg2576 mice is persistent under diet-induced obesity. Int J Mol Sci 23(21).https://doi.org/10.3390/ijms232113591.
Cisternas P, Zolezzi JM, Martinez M, Torres VI, Wong GW, Inestrosa NC (2019) Wnt-induced activation of glucose metabolism mediates the in vivo neuroprotective roles of Wnt signaling in Alzheimer disease. J Neurochem 149(1):54–72. https://doi.org/10.1111/jnc.14608
Tang BL (2020) Glucose, glycolysis, and neurodegenerative diseases. J Cell Physiol 235(11):7653–7662. https://doi.org/10.1002/jcp.29682
Pohanka M (2020) D-lactic acid as a metabolite: toxicology, diagnosis, and detection. Biomed Res Int 2020:3419034. https://doi.org/10.1155/2020/3419034
Proia P, Di Liegro CM, Schiera G, Fricano A, Di Liegro I (2016) Lactate as a metabolite and a regulator in the central nervous system. Int J Mol Sci 17(9). https://doi.org/10.3390/ijms17091450
Brooks GA (2018) The science and translation of lactate shuttle theory. Cell Metab 27(4):757–785. https://doi.org/10.1016/j.cmet.2018.03.008
Dunn J, Grider MH (2023) Physiology, adenosine triphosphate. In: StatPearls. Treasure Island (FL): StatPearls Publishing Copyright © 2023, StatPearls Publishing LLC
DeBerardinis RJ, Mancuso A, Daikhin E, Nissim I, Yudkoff M, Wehrli S, Thompson CB (2007) Beyond aerobic glycolysis: transformed cells can engage in glutamine metabolism that exceeds the requirement for protein and nucleotide synthesis. Proc Natl Acad Sci U S A 104(49):19345–19350. https://doi.org/10.1073/pnas.0709747104
Rabinowitz JD, Enerbäck S (2020) Lactate: the ugly duckling of energy metabolism. Nat Metab 2(7):566–571. https://doi.org/10.1038/s42255-020-0243-4
Maciolek JA, Pasternak JA, Wilson HL (2014) Metabolism of activated T lymphocytes. Curr Opin Immunol 27:60–74. https://doi.org/10.1016/j.coi.2014.01.006
van Hall G (2010) Lactate kinetics in human tissues at rest and during exercise. Acta Physiol (Oxf) 199(4):499–508. https://doi.org/10.1111/j.1748-1716.2010.02122.x
Pellerin L, Magistretti PJ (2012) Sweet sixteen for ANLS. J Cereb Blood Flow Metab 32(7):1152–1166. https://doi.org/10.1038/jcbfm.2011.149
Bennis Y, Bodeau S, Batteux B, Gras-Champel V, Masmoudi K, Maizel J, De Broe ME, Lalau JD et al (2020) A study of associations between plasma metformin concentration, lactic acidosis, and mortality in an emergency hospitalization context. Crit Care Med 48(12):e1194–e1202. https://doi.org/10.1097/ccm.0000000000004589
Brooks GA (2009) Cell-cell and intracellular lactate shuttles. J Physiol 587(Pt 23):5591–5600. https://doi.org/10.1113/jphysiol.2009.178350
Halestrap AP (2013) The SLC16 gene family - structure, role and regulation in health and disease. Mol Aspects Med 34(2–3):337–349. https://doi.org/10.1016/j.mam.2012.05.003
Bergersen LH (2007) Is lactate food for neurons? Comparison of monocarboxylate transporter subtypes in brain and muscle. Neuroscience 145(1):11–19. https://doi.org/10.1016/j.neuroscience.2006.11.062
Magistretti PJ, Allaman I (2018) Lactate in the brain: from metabolic end-product to signalling molecule. Nat Rev Neurosci 19(4):235–249. https://doi.org/10.1038/nrn.2018.19
Gertz EW, Wisneski JA, Stanley WC, Neese RA (1988) Myocardial substrate utilization during exercise in humans. Dual carbon-labeled carbohydrate isotope experiments. J Clin Invest 82(6):2017–2025. https://doi.org/10.1172/jci113822
Rasmussen P, Nielsen J, Overgaard M, Krogh-Madsen R, Gjedde A, Secher NH, Petersen NC (2010) Reduced muscle activation during exercise related to brain oxygenation and metabolism in humans. J Physiol 588(Pt 11):1985–1995. https://doi.org/10.1113/jphysiol.2009.186767
Suzuki A, Stern SA, Bozdagi O, Huntley GW, Walker RH, Magistretti PJ, Alberini CM (2011) Astrocyte-neuron lactate transport is required for long-term memory formation. Cell 144(5):810–823. https://doi.org/10.1016/j.cell.2011.02.018
Gordon GR, Choi HB, Rungta RL, Ellis-Davies GC, MacVicar BA (2008) Brain metabolism dictates the polarity of astrocyte control over arterioles. Nature 456(7223):745–749. https://doi.org/10.1038/nature07525
Xue X, Liu B, Hu J, Bian X, Lou S (2022) The potential mechanisms of lactate in mediating exercise-enhanced cognitive function: a dual role as an energy supply substrate and a signaling molecule. Nutr Metab (Lond) 19(1):52. https://doi.org/10.1186/s12986-022-00687-z
Newman LA, Korol DL, Gold PE (2011) Lactate produced by glycogenolysis in astrocytes regulates memory processing. PLoS ONE 6(12):e28427. https://doi.org/10.1371/journal.pone.0028427
Bouzier-Sore AK, Voisin P, Bouchaud V, Bezancon E, Franconi JM, Pellerin L (2006) Competition between glucose and lactate as oxidative energy substrates in both neurons and astrocytes: a comparative NMR study. Eur J Neurosci 24(6):1687–1694. https://doi.org/10.1111/j.1460-9568.2006.05056.x
Pierre K, Pellerin L (2005) Monocarboxylate transporters in the central nervous system: distribution, regulation and function. J Neurochem 94(1):1–14. https://doi.org/10.1111/j.1471-4159.2005.03168.x
Bélanger M, Allaman I, Magistretti PJ (2011) Brain energy metabolism: focus on astrocyte-neuron metabolic cooperation. Cell Metab 14(6):724–738. https://doi.org/10.1016/j.cmet.2011.08.016
Magistretti PJ, Allaman I (2015) A cellular perspective on brain energy metabolism and functional imaging. Neuron 86(4):883–901. https://doi.org/10.1016/j.neuron.2015.03.035
Medel V, Crossley N, Gajardo I, Muller E, Barros LF, Shine JM, Sierralta J (2022) Whole-brain neuronal MCT2 lactate transporter expression links metabolism to human brain structure and function. Proc Natl Acad Sci U S A 119(33):e2204619119. https://doi.org/10.1073/pnas.2204619119
Matsui T, Ishikawa T, Ito H, Okamoto M, Inoue K, Lee MC, Fujikawa T, Ichitani Y et al (2012) Brain glycogen supercompensation following exhaustive exercise. J Physiol 590(3):607–616. https://doi.org/10.1113/jphysiol.2011.217919
Gibbs ME (2015) Role of glycogenolysis in memory and learning: regulation by noradrenaline, serotonin and ATP. Front Integr Neurosci 9:70. https://doi.org/10.3389/fnint.2015.00070
Kerendi H, Rahmati M, Mirnasuri R, Kazemi A (2019) High intensity interval training decreases the expressions of KIF5B and dynein in hippocampus of Wistar male rats. Gene 704:8–14. https://doi.org/10.1016/j.gene.2019.04.027
Sada N, Lee S, Katsu T, Otsuki T, Inoue T (2015) Epilepsy treatment. Targeting LDH enzymes with a stiripentol analog to treat epilepsy. Science 347(6228):1362–1367. https://doi.org/10.1126/science.aaa1299
Rahmati M, Kazemi A (2019) Various exercise intensities differentially regulate GAP-43 and CAP-1 expression in the rat hippocampus. Gene 692:185–194. https://doi.org/10.1016/j.gene.2019.01.013
Sole G (2015) ESSA’s student manual for health, exercise and sport assessment (2014). N Z J Physiother 43(1):33
Faude O, Kindermann W, Meyer T (2009) Lactate threshold concepts: how valid are they? Sports Med 39(6):469–490. https://doi.org/10.2165/00007256-200939060-00003
Jamnick NA, Pettitt RW, Granata C, Pyne DB, Bishop DJ (2020) An examination and critique of current methods to determine exercise intensity. Sports Med 50(10):1729–1756. https://doi.org/10.1007/s40279-020-01322-8
Goodman JC, Valadka AB, Gopinath SP, Uzura M, Robertson CS (1999) Extracellular lactate and glucose alterations in the brain after head injury measured by microdialysis. Crit Care Med 27(9):1965–1973. https://doi.org/10.1097/00003246-199909000-00041
Harada M, Okuda C, Sawa T, Murakami T (1992) Cerebral extracellular glucose and lactate concentrations during and after moderate hypoxia in glucose- and saline-infused rats. Anesthesiology 77(4):728–734. https://doi.org/10.1097/00000542-199210000-00017
Boumezbeur F, Petersen KF, Cline GW, Mason GF, Behar KL, Shulman GI, Rothman DL (2010) The contribution of blood lactate to brain energy metabolism in humans measured by dynamic 13C nuclear magnetic resonance spectroscopy. J Neurosci 30(42):13983–13991. https://doi.org/10.1523/jneurosci.2040-10.2010
Lucertini F, Gervasi M, D’Amen G, Sisti D, Rocchi MBL, Stocchi V, Benelli P (2017) Effect of water-based recovery on blood lactate removal after high-intensity exercise. PLoS ONE 12(9):e0184240. https://doi.org/10.1371/journal.pone.0184240
Gastin PB (2001) Energy system interaction and relative contribution during maximal exercise. Sports Med 31(10):725–741. https://doi.org/10.2165/00007256-200131100-00003
Brooks GA, Butterfield GE, Wolfe RR, Groves BM, Mazzeo RS, Sutton JR, Wolfel EE (1991) Decreased reliance on lactate during exercise after acclimatization to 4,300 m. J Appl Physiol (1985) 71(1):333–341. https://doi.org/10.1152/jappl.1991.71.1.333
De Feo P, Di Loreto C, Lucidi P, Murdolo G, Parlanti N, De Cicco A, Piccioni F, Santeusanio F (2003) Metabolic response to exercise. J Endocrinol Invest 26(9):851–854. https://doi.org/10.1007/bf03345235
Rahmati M, Shariatzadeh Joneydi M, Koyanagi A, Yang G, Ji B, Won Lee S, Keon Yon D et al (2023) Resistance training restores skeletal muscle atrophy and satellite cell content in an animal model of Alzheimer’s disease. Sci Rep 13(1):2535. https://doi.org/10.1038/s41598-023-29406-1
Téglás T, Ábrahám D, Jókai M, Kondo S, Mohammadi R, Fehér J, Szabó D, Wilhelm M et al (2020) Exercise combined with a probiotics treatment alters the microbiome, but moderately affects signalling pathways in the liver of male APP/PS1 transgenic mice. Biogerontology 21(6):807–815. https://doi.org/10.1007/s10522-020-09895-7
Hötting K, Röder B (2013) Beneficial effects of physical exercise on neuroplasticity and cognition. Neurosci Biobehav Rev 37(9 Pt B):2243–2257. https://doi.org/10.1016/j.neubiorev.2013.04.005
Shen Z, Jiang L, Yuan Y, Deng T, Zheng YR, Zhao YY, Li WL, Wu JY et al (2015) Inhibition of G protein-coupled receptor 81 (GPR81) protects against ischemic brain injury. CNS Neurosci Ther 21(3):271–279. https://doi.org/10.1111/cns.12362
Li G, Wang HQ, Wang LH, Chen RP, Liu JP (2014) Distinct pathways of ERK1/2 activation by hydroxy-carboxylic acid receptor-1. PLoS ONE 9(3):e93041. https://doi.org/10.1371/journal.pone.0093041
Jeninga EH, Bugge A, Nielsen R, Kersten S, Hamers N, Dani C, Wabitsch M, Berger R et al (2009) Peroxisome proliferator-activated receptor gamma regulates expression of the anti-lipolytic G-protein-coupled receptor 81 (GPR81/Gpr81). J Biol Chem 284(39):26385–26393. https://doi.org/10.1074/jbc.M109.040741
Madaan A, Chaudhari P, Nadeau-Vallée M, Hamel D, Zhu T, Mitchell G, Samuels M, Pundir S et al (2019) Müller cell-localized G-protein-coupled receptor 81 (hydroxycarboxylic acid receptor 1) regulates inner retinal vasculature via norrin/Wnt pathways. Am J Pathol 189(9):1878–1896. https://doi.org/10.1016/j.ajpath.2019.05.016
Seifert T, Brassard P, Wissenberg M, Rasmussen P, Nordby P, Stallknecht B, Adser H, Jakobsen AH et al (2010) Endurance training enhances BDNF release from the human brain. Am J Physiol Regul Integr Comp Physiol 298(2):R372-377. https://doi.org/10.1152/ajpregu.00525.2009
Inoue K, Okamoto M, Shibato J, Lee MC, Matsui T, Rakwal R, Soya H (2015) Long-term mild, rather than intense, exercise enhances adult hippocampal neurogenesis and greatly changes the transcriptomic profile of the hippocampus. PLoS ONE 10(6):e0128720. https://doi.org/10.1371/journal.pone.0128720
Skriver K, Roig M, Lundbye-Jensen J, Pingel J, Helge JW, Kiens B, Nielsen JB (2014) Acute exercise improves motor memory: exploring potential biomarkers. Neurobiol Learn Mem 116:46–58. https://doi.org/10.1016/j.nlm.2014.08.004
Di Benedetto G, Iannucci LF, Surdo NC, Zanin S, Conca F, Grisan F, Gerbino A, Lefkimmiatis K (2021) Compartmentalized signaling in aging and neurodegeneration. Cells 10(2). https://doi.org/10.3390/cells10020464
Yang J, Ruchti E, Petit JM, Jourdain P, Grenningloh G, Allaman I, Magistretti PJ (2014) Lactate promotes plasticity gene expression by potentiating NMDA signaling in neurons. Proc Natl Acad Sci U S A 111(33):12228–12233. https://doi.org/10.1073/pnas.1322912111
Wang M, Gamo NJ, Yang Y, Jin LE, Wang XJ, Laubach M, Mazer JA, Lee D et al (2011) Neuronal basis of age-related working memory decline. Nature 476(7359):210–213. https://doi.org/10.1038/nature10243
Kamat PK, Kalani A, Rai S, Swarnkar S, Tota S, Nath C, Tyagi N (2016) Mechanism of oxidative stress and synapse dysfunction in the pathogenesis of Alzheimer’s disease: understanding the therapeutics strategies. Mol Neurobiol 53(1):648–661. https://doi.org/10.1007/s12035-014-9053-6
Morland C, Lauritzen KH, Puchades M, Holm-Hansen S, Andersson K, Gjedde A, Attramadal H, Storm-Mathisen J et al (2015) The lactate receptor, G-protein-coupled receptor 81/hydroxycarboxylic acid receptor 1: expression and action in brain. J Neurosci Res 93(7):1045–1055. https://doi.org/10.1002/jnr.23593
Roskoski R Jr (2012) ERK1/2 MAP kinases: structure, function, and regulation. Pharmacol Res 66(2):105–143. https://doi.org/10.1016/j.phrs.2012.04.005
Hu J, Cai M, Liu Y, Liu B, Xue X, Ji R, Bian X, Lou S (2020) The roles of GRP81 as a metabolic sensor and inflammatory mediator. J Cell Physiol 235(12):8938–8950. https://doi.org/10.1002/jcp.29739
Fallah Mohammadi Z, Falah Mohammadi H, Patel DI (2019) Comparing the effects of progressive and mild intensity treadmill running protocols on neuroprotection of parkinsonian rats. Life Sci 229:219–224. https://doi.org/10.1016/j.lfs.2019.05.036
Oh S, Seo SB, Kim G, Batsukh S, Son KH, Byun K (2023) Poly-D,L-lactic acid stimulates angiogenesis and collagen synthesis in aged animal skin. Int J Mol Sci 24(9). https://doi.org/10.3390/ijms24097986
Moonen S, Koper MJ, Van Schoor E, Schaeverbeke JM, Vandenberghe R, von Arnim CAF, Tousseyn T, De Strooper B et al (2023) Pyroptosis in Alzheimer’s disease: cell type-specific activation in microglia, astrocytes and neurons. Acta Neuropathol 145(2):175–195. https://doi.org/10.1007/s00401-022-02528-y
Végran F, Boidot R, Michiels C, Sonveaux P, Feron O (2011) Lactate influx through the endothelial cell monocarboxylate transporter MCT1 supports an NF-κB/IL-8 pathway that drives tumor angiogenesis. Cancer Res 71(7):2550–2560. https://doi.org/10.1158/0008-5472.Can-10-2828
Rooney K, Trayhurn P (2011) Lactate and the GPR81 receptor in metabolic regulation: implications for adipose tissue function and fatty acid utilisation by muscle during exercise. Br J Nutr 106(9):1310–1316. https://doi.org/10.1017/s0007114511004673
Lin HC, Chen YJ, Wei YH, Lin HA, Chen CC, Liu TF, Hsieh YL, Huang KY et al (2021) Lactic acid fermentation is required for NLRP3 inflammasome activation. Front Immunol 12:630380. https://doi.org/10.3389/fimmu.2021.630380
Yang K, Xu J, Fan M, Tu F, Wang X, Ha T, Williams DL, Li C (2020) Lactate suppresses macrophage pro-inflammatory response to LPS stimulation by inhibition of YAP and NF-κB activation via GPR81-mediated signaling. Front Immunol 11:587913. https://doi.org/10.3389/fimmu.2020.587913
Nasi A, Fekete T, Krishnamurthy A, Snowden S, Rajnavölgyi E, Catrina AI, Wheelock CE, Vivar N et al (2013) Dendritic cell reprogramming by endogenously produced lactic acid. J Immunol 191(6):3090–3099. https://doi.org/10.4049/jimmunol.1300772
Hoque R, Farooq A, Ghani A, Gorelick F, Mehal WZ (2014) Lactate reduces liver and pancreatic injury in Toll-like receptor- and inflammasome-mediated inflammation via GPR81-mediated suppression of innate immunity. Gastroenterology 146(7):1763–1774. https://doi.org/10.1053/j.gastro.2014.03.014
Santoni G, Cardinali C, Morelli MB, Santoni M, Nabissi M, Amantini C (2015) Danger- and pathogen-associated molecular patterns recognition by pattern-recognition receptors and ion channels of the transient receptor potential family triggers the inflammasome activation in immune cells and sensory neurons. J Neuroinflammation 12:21. https://doi.org/10.1186/s12974-015-0239-2
Manosalva C, Quiroga J, Hidalgo AI, Alarcón P, Anseoleaga N, Hidalgo MA, Burgos RA (2021) Role of lactate in inflammatory processes: friend or foe. Front Immunol 12:808799. https://doi.org/10.3389/fimmu.2021.808799
Yang L (2018) Neuronal cAMP/PKA signaling and energy homeostasis. Adv Exp Med Biol 1090:31–48. https://doi.org/10.1007/978-981-13-1286-1_3
Omar MH, Scott JD (2020) AKAP signaling islands: venues for precision pharmacology. Trends Pharmacol Sci 41(12):933–946. https://doi.org/10.1016/j.tips.2020.09.007
Keravis T, Lugnier C (2012) Cyclic nucleotide phosphodiesterase (PDE) isozymes as targets of the intracellular signalling network: benefits of PDE inhibitors in various diseases and perspectives for future therapeutic developments. Br J Pharmacol 165(5):1288–1305. https://doi.org/10.1111/j.1476-5381.2011.01729.x
Zhou Z, Okamoto K, Onodera J, Hiragi T, Andoh M, Ikawa M, Tanaka KF, Ikegaya Y, et al. (2021) Astrocytic cAMP modulates memory via synaptic plasticity. Proc Natl Acad Sci USA 118(3). https://doi.org/10.1073/pnas.2016584118
Liu X, Betzenhauser MJ, Reiken S, Meli AC, Xie W, Chen BX, Arancio O, Marks AR (2012) Role of leaky neuronal ryanodine receptors in stress-induced cognitive dysfunction. Cell 150(5):1055–1067. https://doi.org/10.1016/j.cell.2012.06.052
Toledo JB, Arnold SE, Raible K, Brettschneider J, Xie SX, Grossman M, Monsell SE, Kukull WA et al (2013) Contribution of cerebrovascular disease in autopsy confirmed neurodegenerative disease cases in the National Alzheimer’s Coordinating Centre. Brain 136(Pt 9):2697–2706. https://doi.org/10.1093/brain/awt188
Radeva MY, Waschke J (2018) Mind the gap: mechanisms regulating the endothelial barrier. Acta Physiol (Oxf) 222(1) https://doi.org/10.1111/apha.12860
Viña D, Seoane N, Vasquez EC, Campos-Toimil M (2021) cAMP compartmentalization in cerebrovascular endothelial cells: new therapeutic opportunities in Alzheimer’s disease. Cells 10(8). https://doi.org/10.3390/cells10081951
Lauritzen KH, Morland C, Puchades M, Holm-Hansen S, Hagelin EM, Lauritzen F, Attramadal H, Storm-Mathisen J et al (2014) Lactate receptor sites link neurotransmission, neurovascular coupling, and brain energy metabolism. Cereb Cortex 24(10):2784–2795. https://doi.org/10.1093/cercor/bht136
Ivashkiv LB (2020) The hypoxia-lactate axis tempers inflammation. Nat Rev Immunol 20(2):85–86. https://doi.org/10.1038/s41577-019-0259-8
Bozzo L, Puyal J, Chatton JY (2013) Lactate modulates the activity of primary cortical neurons through a receptor-mediated pathway. PLoS ONE 8(8):e71721. https://doi.org/10.1371/journal.pone.0071721
de Castro AH, Briquet M, Schmuziger C, Restivo L, Puyal J, Rosenberg N, Rocher AB, Offermanns S et al (2019) The lactate receptor HCAR1 modulates neuronal network activity through the activation of G(α) and G(βγ) subunits. J Neurosci 39(23):4422–4433. https://doi.org/10.1523/jneurosci.2092-18.2019
Booker SA, Wyllie DJA (2021) NMDA receptor function in inhibitory neurons. Neuropharmacology 196:108609. https://doi.org/10.1016/j.neuropharm.2021.108609
Paoletti P, Bellone C, Zhou Q (2013) NMDA receptor subunit diversity: impact on receptor properties, synaptic plasticity and disease. Nat Rev Neurosci 14(6):383–400. https://doi.org/10.1038/nrn3504
Hynd MR, Scott HL, Dodd PR (2004) Glutamate-mediated excitotoxicity and neurodegeneration in Alzheimer’s disease. Neurochem Int 45(5):583–595. https://doi.org/10.1016/j.neuint.2004.03.007
Bezprozvanny I, Mattson MP (2008) Neuronal calcium mishandling and the pathogenesis of Alzheimer’s disease. Trends Neurosci 31(9):454–463. https://doi.org/10.1016/j.tins.2008.06.005
Herrera-López G, Griego E, Galván EJ (2020) Lactate induces synapse-specific potentiation on CA3 pyramidal cells of rat hippocampus. PLoS ONE 15(11):e0242309. https://doi.org/10.1371/journal.pone.0242309
Mosienko V, Teschemacher AG, Kasparov S (2015) Is L-lactate a novel signaling molecule in the brain? J Cereb Blood Flow Metab 35(7):1069–1075. https://doi.org/10.1038/jcbfm.2015.77
Wang J, Cui Y, Yu Z, Wang W, Cheng X, Ji W, Guo S, Zhou Q et al (2019) Brain endothelial cells maintain lactate homeostasis and control adult hippocampal neurogenesis. Cell Stem Cell 25(6):754-767.e759. https://doi.org/10.1016/j.stem.2019.09.009
Steinman MQ, Gao V, Alberini CM (2016) The role of lactate-mediated metabolic coupling between astrocytes and neurons in long-term memory formation. Front Integr Neurosci 10:10. https://doi.org/10.3389/fnint.2016.00010
Descalzi G, Gao V, Steinman MQ, Suzuki A, Alberini CM (2019) Lactate from astrocytes fuels learning-induced mRNA translation in excitatory and inhibitory neurons. Commun Biol 2:247. https://doi.org/10.1038/s42003-019-0495-2
Lu WT, Sun SQ, Li Y, Xu SY, Gan SW, Xu J, Qiu GP, Zhuo F et al (2019) Curcumin ameliorates memory deficits by enhancing lactate content and MCT2 expression in APP/PS1 transgenic mouse model of Alzheimer’s disease. Anat Rec (Hoboken) 302(2):332–338. https://doi.org/10.1002/ar.23969
Liguori C, Chiaravalloti A, Sancesario G, Stefani A, Sancesario GM, Mercuri NB, Schillaci O, Pierantozzi M (2016) Cerebrospinal fluid lactate levels and brain [18F]FDG PET hypometabolism within the default mode network in Alzheimer’s disease. Eur J Nucl Med Mol Imaging 43(11):2040–2049. https://doi.org/10.1007/s00259-016-3417-2
Hong P, Zhang X, Gao S, Wang P (2020) Role of monocarboxylate transporter 4 in Alzheimer disease. Neurotoxicology 76:191–199. https://doi.org/10.1016/j.neuro.2019.11.006
El Hayek L, Khalifeh M, Zibara V, Abi Assaad R, Emmanuel N, Karnib N, El-Ghandour R, Nasrallah P et al (2019) Lactate mediates the effects of exercise on learning and memory through SIRT1-dependent activation of hippocampal brain-derived neurotrophic factor (BDNF). J Neurosci 39(13):2369–2382. https://doi.org/10.1523/jneurosci.1661-18.2019
Veloz Castillo MF, Magistretti PJ, Calì C (2021) L-lactate: food for thoughts, memory and behavior. Metabolites 11(8). https://doi.org/10.3390/metabo11080548
Shima T, Matsui T, Jesmin S, Okamoto M, Soya M, Inoue K, Liu YF, Torres-Aleman I et al (2017) Moderate exercise ameliorates dysregulated hippocampal glycometabolism and memory function in a rat model of type 2 diabetes. Diabetologia 60(3):597–606. https://doi.org/10.1007/s00125-016-4164-4
Berthet C, Lei H, Thevenet J, Gruetter R, Magistretti PJ, Hirt L (2009) Neuroprotective role of lactate after cerebral ischemia. J Cereb Blood Flow Metab 29(11):1780–1789. https://doi.org/10.1038/jcbfm.2009.97
Castillo X, Rosafio K, Wyss MT, Drandarov K, Buck A, Pellerin L, Weber B, Hirt L (2015) A probable dual mode of action for both L- and D-lactate neuroprotection in cerebral ischemia. J Cereb Blood Flow Metab 35(10):1561–1569. https://doi.org/10.1038/jcbfm.2015.115
Kennedy L, Glesaaen ER, Palibrk V, Pannone M, Wang W, Al-Jabri A, Suganthan R, Meyer N, et al (2022) Lactate receptor HCAR1 regulates neurogenesis and microglia activation after neonatal hypoxia-ischemia. Elife 11. https://doi.org/10.7554/eLife.76451
Lu W, Huang J, Sun S, Huang S, Gan S, Xu J, Yang M, Xu S et al (2015) Changes in lactate content and monocarboxylate transporter 2 expression in Aβ25-35-treated rat model of Alzheimer’s disease. Neurol Sci 36(6):871–876. https://doi.org/10.1007/s10072-015-2087-3
Zhang M, Cheng X, Dang R, Zhang W, Zhang J, Yao Z (2018) Lactate deficit in an alzheimer disease mouse model: the relationship with neuronal damage. J Neuropathol Exp Neurol 77(12):1163–1176. https://doi.org/10.1093/jnen/nly102
Cai M, Wang H, Song H, Yang R, Wang L, Xue X, Sun W, Hu J (2022) Lactate is answerable for brain function and treating brain diseases: energy substrates and signal molecule. Front Nutr 9:800901. https://doi.org/10.3389/fnut.2022.800901
Redjems-Bennani N, Jeandel C, Lefebvre E, Blain H, Vidailhet M, Guéant JL (1998) Abnormal substrate levels that depend upon mitochondrial function in cerebrospinal fluid from Alzheimer patients. Gerontology 44(5):300–304. https://doi.org/10.1159/000022031
Fernandez-Marcos PJ, Auwerx J (2011) Regulation of PGC-1α, a nodal regulator of mitochondrial biogenesis. Am J Clin Nutr 93(4):884s–8890. https://doi.org/10.3945/ajcn.110.001917
E L, Lu J, Selfridge JE, Burns JM, Swerdlow RH (2013) Lactate administration reproduces specific brain and liver exercise-related changes. J Neurochem 127(1):91–100. https://doi.org/10.1111/jnc.12394
Laurin D, Verreault R, Lindsay J, MacPherson K, Rockwood K (2001) Physical activity and risk of cognitive impairment and dementia in elderly persons. Arch Neurol 58(3):498–504. https://doi.org/10.1001/archneur.58.3.498
De la Rosa A, Olaso-Gonzalez G, Arc-Chagnaud C, Millan F, Salvador-Pascual A, Garcia-Lucerga C, Blasco-Lafarga C, Garcia-Dominguez E et al (2020) Physical exercise in the prevention and treatment of Alzheimer’s disease. J Sport Health Sci 9(5):394–404. https://doi.org/10.1016/j.jshs.2020.01.004
Lee IM, Shiroma EJ, Lobelo F, Puska P, Blair SN, Katzmarzyk PT (2012) Effect of physical inactivity on major non-communicable diseases worldwide: an analysis of burden of disease and life expectancy. Lancet 380(9838):219–229. https://doi.org/10.1016/s0140-6736(12)61031-9
Funding
This study was funded by the National Natural Science Foundation of China (Grant No. 81702236), Hunan Provincial Natural Science Foundation (Approval No. 2023JJ30429), Changsha City Natural Science Foundation (Grant No. kq2202251), and Key Project of the Hunan Provincial Education Department (Grant No. 20A333).
Author information
Authors and Affiliations
Contributions
Xiangyuan Meng and Weijia Wu are the co-first authors. They drafted the manuscript and contributed equally to this article. Yingzhe Tang and Mei Peng drafted the figures. Shunling Yuan and Zelin Hu suggested improvements. Wenfeng Liu conceptualized the article and revised the final version of the manuscript. All the authors have read and approved the final version of the manuscript.
Corresponding author
Ethics declarations
Ethics Approval
Not applicable.
Consent to Participate
Not applicable.
Consent for Publication
Not applicable.
Competing Interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Meng, X., Wu, W., Tang, Y. et al. Lactate/Hydroxycarboxylic Acid Receptor 1 in Alzheimer’s Disease: Mechanisms and Therapeutic Implications-Exercise Perspective. Mol Neurobiol (2024). https://doi.org/10.1007/s12035-024-04067-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12035-024-04067-x