Abstract
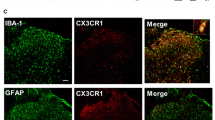
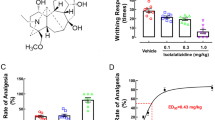
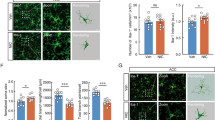
Injection of polyinosinic:polycytidylic acid (poly(I:C)) into experimental animals induces neuroimmunological responses and thus has been used for the study of neurological disorders such as anxiety, depression, and chronic fatigue. Here, we investigated the effects of vagus nerve stimulation (VNS) on poly(I:C)-induced neuroinflammation and associated behavioral consequences in rats. The microglia in the prefrontal cortex (PFC) displayed the activated form of morphology in poly(I:C)-injected rats and changed to a normal shape after acute VNS (aVNS). Production of phospho-NF-κB, phospho-IκB, IL-1β, and cleaved caspase 3 was elevated by poly(I:C) and downregulated by aVNS. In contrast, phospho-Akt levels were decreased by poly(I:C) and increased by aVNS. Neuronal production of fractalkine (CX3CL1) in the PFC was markedly reduced by poly(I:C), but recovered by aVNS. Fractalkine interaction with its receptor CX3CR1 was highly elevated by VNS. We further demonstrated that the pharmacological blockade of CX3CR1 activity counteracted the production of IL-1β, phospho-Akt, and cleaved form of caspase 3 that was modulated by VNS, suggesting the anti-inflammatory effects of fractalkine-CX3CR1 signaling as a mediator of neuron-microglia interaction. Behavioral assessments of pain and temperature sensations by von Frey and hot/cold plate tests showed significant improvement by chronic VNS (cVNS) and forced swimming and marble burying tests revealed that the depressive-like behaviors caused by poly(I:C) injection were rescued by cVNS. We also found that the recognition memory which was impaired by poly(I:C) administration was improved by cVNS. This study suggests that VNS may play a role in regulating neuroinflammation and somatosensory and cognitive functions in poly(I:C)-injected animals.







Similar content being viewed by others
Data Availability
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- aVNS:
-
Acute vagus nerve stimulation
- CFS:
-
Chronic fatigue syndrome
- cVNS:
-
Chronic vagus nerve stimulation
- FST:
-
Forced swimming test
- LPS:
-
Lipopolysaccharide
- PFC:
-
Prefrontal cortex
- poly(I:C):
-
Polyinosinic:polycytidylic acid
- VNS:
-
Vagus nerve stimulation
References
Alexopoulou L, Holt AC, Medzhitov R, Flavell RA (2001) Recognition of double-stranded RNA and activation of NF-kappaB by Toll-like receptor 3. Nature 413(6857):732–738. https://doi.org/10.1038/35099560
Murshid A, Borges TJ, Lang BJ, Calderwood SK (2016) The scavenger receptor SREC-I cooperates with toll-like receptors to trigger inflammatory innate immune responses. Front Immunol 7:226. https://doi.org/10.3389/fimmu.2016.00226
Scumpia PO, Kelly KM, Reeves WH, Stevens BR (2005) Double-stranded RNA signals antiviral and inflammatory programs and dysfunctional glutamate transport in TLR3-expressing astrocytes. Glia 52(2):153–162. https://doi.org/10.1002/glia.20234
Ifuku M, Hossain SM, Noda M, Katafuchi T (2014) Induction of interleukin1beta by activated microglia is a prerequisite for immunologically induced fatigue. Eur J Neurosci 40:3253–3263. https://doi.org/10.1111/ejn.12668
Lee H, Park C, Cho IH, Kim HY, Jo EK, Lee S et al (2007) Double-stranded RNA induces iNOS gene expression in Schwann cells, sensory neuronal death, and peripheral nerve demyelination. Glia 55(7):712–722. https://doi.org/10.1002/glia.20493
Garré JM, Silva HM, Lafaille JJ, Yang G (2017) CX3CR1+ monocytes modulate learning and learning-dependent dendritic spine remodeling via TNF-α. Nat Med 23(6):714–722. https://doi.org/10.1038/nm.4340
Reisinger S, Khan D, Kong E, Berger A, Pollak A, Pollak DD (2015) The poly(I:C)-induced maternal immune activation model in preclinical neuropsychiatric drug discovery. Pharmacol Ther 149:213–226. https://doi.org/10.1016/j.pharmthera.2015.01.001
Chambers D, Bagnall AM, Hempel S, Forbes C (2006) Interventions for the treatment, management and rehabilitation of patients with chronic fatigue syndrome/myalgic encephalomyelitis: an updated systematic review. J R Soc Med 99(10):506–520. https://doi.org/10.1177/014107680609901012
Komaroff AL, Takahashi R, Yamamura T, Sawamura M (2018) Neurologic abnormalities in myalgic encephalomyelitis/chronic fatigue syndrome: a review. Brain Nerve 70(1):41–54. https://doi.org/10.11477/mf.1416200948
Cleare AJ (2003) The neuroendocrinology of chronic fatigue syndrome. Endocr Rev 24:236–252. https://doi.org/10.1210/er.2002-0014
Lorusso L, Mikhaylova SV, Capelli E, Ferrari D, Ngonga GK, Ricevuti G (2009) Immunological aspects of chronic fatigue syndrome. Autoimmun Rev 8:287–291. https://doi.org/10.1016/j.autrev.2008.08.003
Cambras T, Castro-Marrero J, Zaragoza MC, Diez-Noguera A, Alegre J (2018) Circadian rhythm abnormalities and autonomic dysfunction in patients with chronic fatigue syndrome/myalgic encephalomyelitis. PLoS One 13:e0198106. https://doi.org/10.1371/journal.pone.0198106
Morris G, Stubbs B, Kohler CA, Walder K, Slyepchenko A, Berk M et al (2018) The putative role of oxidative stress and inflammation in the pathophysiology of sleep dysfunction across neuropsychiatric disorders: focus on chronic fatigue syndrome, bipolar disorder and multiple sclerosis. Sleep Med Rev 41:255–265. https://doi.org/10.1016/j.smrv.2018.03.007
Noda M, Ifuku M, Hossain MS, Glial KT (2018) Activation and expression of the serotonin transporter in chronic fatigue syndrome. Front Psychiatry 9:589. https://doi.org/10.3389/fpsyt.2018.00589
Nakatomi Y, Mizuno K, Ishii A, Wada Y, Tanaka M, Tazawa S et al (2014) Neuroinflammation in patients with chronic fatigue syndrome/myalgic encephalomyelitis: an (1)(1)C-(R)-PK11195 PET study. J Nucl Med 55:945–50. https://doi.org/10.11477/mf.1416200945
Han W, Tellez LA, Perkins MH, Perez IO, Qu T, Ferreira J et al (2018) A neural circuit for gut-induced reward. Cell 175(3):665–678. https://doi.org/10.1016/j.cell.2018.10.018
Furmaga H, Carreno FR, Frazer A (2012) Vagal nerve stimulation rapidly activates brain-derived neurotrophic factor receptor TrkB in rat brain. PLoS One 7(5):e34844. https://doi.org/10.1371/journal.pone.0034844
Shin HC, Jo BG, Lee CY, Lee KW, Namgung U (2019) Hippocampal activation of 5-HT1B receptors and BDNF production by vagus nerve stimulation in rats under chronic restraint stress. Eur J Neurosci 50(1):1820–1830. https://doi.org/10.1016/j.neuroscience.2016.02.024
Shah AP, Carreno FR, Wu H, Chung YA, Frazer A (2016) Role of TrkB in the anxiolytic-like and antidepressant-like effects of vagal nerve stimulation: comparison with desipramine. Neuroscience 322:273–286
Wang Y, Zhan G, Cai Z, Jiao B, Zhao Y, Li S, Luo A (2021) Vagus nerve stimulation in brain diseases: therapeutic applications and biological mechanisms. Neurosci Biobehav Rev 127:37–53. https://doi.org/10.1016/j.neubiorev.2021.04.018
Chen G, Zhou Z, Sha W, Wang L, Yan F, Yang X et al (2020) A novel CX3CR1 inhibitor AZD8797 facilitates early recovery of rat acute spinal cord injury by inhibiting inflammation and apoptosis. Int J Mol Med 45(5):1373–1384. https://doi.org/10.3892/ijmm.2020.4509
Namgung U, Kim KJ, Jo BG, Park JM (2022) Vagus nerve stimulation modulates hippocampal inflammation caused by continuous stress in rats. J Neuroinflammation 19(1):33. https://doi.org/10.1186/s12974-022-02396-z
Jo BG, Kim SH, Namgung U (2020) Vagal afferent fibers contribute to the anti-inflammatory reactions by vagus nerve stimulation in concanavalin A model of hepatitis in rats. Mol Med 26(1):119. https://doi.org/10.1186/s10020-020-00247-2
Hwang J, Namgung U (2021) Phosphorylation of STAT3 by axonal Cdk5 promotes axonal regeneration by modulating mitochondrial activity. Exp Neurol 335:113511. https://doi.org/10.1016/j.expneurol.2020.113511
Lee KW, Im JY, Song JS, Lee SH, Lee HJ, Ha HY et al (2006) Progressive neuronal loss and behavioral impairments of transgenic C57BL/6 inbred mice expressing the carboxy terminus of amyloid precursor protein. Neurobiol Dis 22(1):10–24. https://doi.org/10.1016/j.nbd.2005.09.011
Leger M, Quiedeville A, Bouet V, Haelewyn B, Boulouard M, Schumann-Bard P, Freret T (2013) Object recognition test in mice. Nat Protoc 8(12):2531–2537. https://doi.org/10.1038/nprot.2013.155
Kim KJ, Hwang J, Park JY, Namgung U (2020) Augmented Buyang Huanwu Decoction facilitates axonal regeneration after peripheral nerve transection through the regulation of inflammatory cytokine production. J Ethnopharmacol 260:113063. https://doi.org/10.1016/j.jep.2020.113063
Bazan JF, Bacon KB, Hardiman G, Wang W, Soo K, Rossi D et al (1997) A new class of membrane-bound chemokine with a CX3C motif. Nature 385(6617):640–644. https://doi.org/10.1038/385640a0
Chamera K, Kotarska K, Szuster-Głuszczak M, Trojan E, Skórkowska A, Pomierny B et al (2020) The prenatal challenge with lipopolysaccharide and polyinosinic:polycytidylic acid disrupts CX3CL1-CX3CR1 and CD200-CD200R signalling in the brains of male rat offspring: a link to schizophrenia-like behaviours. J Neuroinflammation 17(1):247. https://doi.org/10.1186/s12974-020-01923-0
Zhan Y, Paolicelli RC, Sforazzini F, Weinhard L, Bolasco G, Pagani F et al (2014) Deficient neuron-microglia signaling results in impaired functional brain connectivity and social behavior. Nat Neurosci 17(3):400–406. https://doi.org/10.1038/nn.3641
Harrison JK, Jiang Y, Chen S, Xia Y, Maciejewski D, McNamara RK et al (1998) Role for neuronally derived fractalkine in mediating interactions between neurons and CX3CR1-expressing microglia. Proc Natl Acad Sci U S A 95(18):10896–10901. https://doi.org/10.1073/pnas.95.18.10896
Scholz J, Woolf CJ (2007) The neuropathic pain triad: neurons, immune cells and glia. Nat Neurosci 10(11):1361–1368. https://doi.org/10.1038/nn1992
Meucci O, Fatatis A, Simen AA, Miller RJ (2000) Expression of CX3CR1 chemokine receptors on neurons and their role in neuronal survival. Proc Natl Acad Sci U S A 97(14):8075–8080. https://doi.org/10.1073/pnas.090017497
Sheridan GK, Murphy KJ (2013) Neuron-glia crosstalk in health and disease: fractalkine and CX3CR1 take centre stage. Open Biol 3(12):130181. https://doi.org/10.1098/rsob.130181
Hickman S, Izzy S, Sen P, Morsett L, El Khoury J (2018) Microglia in neurodegeneration. Nat Neurosci 21(10):1359–1369. https://doi.org/10.1038/s41593-018-0242-x
Cardona AE, Pioro EP, Sasse ME, Kostenko V, Cardona SM, Dijkstra IM et al (2006) Control of microglial neurotoxicity by the fractalkine receptor. Nat Neurosci 9(7):917–924. https://doi.org/10.1038/nn1715
Subbarayan MS, Joly-Amado A, Bickford PC, Nash KR (2022) CX3CL1/CX3CR1 signaling targets for the treatment of neurodegenerative diseases. Pharmacol Ther 231:107989. https://doi.org/10.1016/j.pharmthera.2021.107989
Yamaguchi T, Yoshimura T, Ohara T, Fujisawa M, Tong G, Matsukawa A (2021) PolyI: C suppresses TGF-β1-induced Akt phosphorylation and reduces the motility of A549 lung carcinoma cells. Mol Biol Rep 48(9):6313–6321. https://doi.org/10.1007/s11033-021-06625-1
Yao X, Qi L, Chen X, Du J, Zhang Z, Liu S (2014) Expression of CX3CR1 associates with cellular migration, metastasis, and prognosis in human clear cell renal cell carcinoma. Urol Oncol 32(2):162–170. https://doi.org/10.1016/j.urolonc.2012.12.006
Lyons A, Lynch AM, Downer EJ, Hanley R, O’Sullivan JB, Smith A et al (2009) Fractalkine-induced activation of the phosphatidylinositol-3 kinase pathway attentuates microglial activation in vivo and in vitro. J Neurochem 110(5):1547–1556. https://doi.org/10.1111/j.1471-4159.2009.06253.x
Lu X, Costantini T, Lopez NE, Wolf PL, Hageny AM, Putnam J et al (2013) Vagal nerve stimulation protects cardiac injury by attenuating mitochondrial dysfunction in a murine burn injury model. J Cell Mol Med 17(5):664–671. https://doi.org/10.1111/jcmm.12049
Carreno FR, Frazer A (2017) Vagal nerve stimulation for treatment-resistant depression. Neurotherapeutics 14(3):716–727. https://doi.org/10.1007/s13311-017-0537-8
Lewis V, Rodrigue B, Arsenault E, Zhang M, Taghavi-Abkuh FF, Silva WCC et al (2023) Translational control by ketamine and its implications for comorbid cognitive deficits in depressive disorders. J Neurochem 166(1):10–23. https://doi.org/10.1111/jnc.15652
Yang Y, Maher DP, Cohen SP (2020) Emerging concepts on the use of ketamine for chronic pain. Expert Rev Clin Pharmacol 13(2):135–146. https://doi.org/10.1080/17512433.2020.1717947
Jett JD, Boley AM, Girotti M, Shah A, Lodge DJ, Morilak DA (2015) Antidepressant-like cognitive and behavioral effects of acute ketamine administration associated with plasticity in the ventral hippocampus to medial prefrontal cortex pathway. Psychopharmacology 232(17):3123–3133. https://doi.org/10.1007/s00213-015-3957-3
Papp M, Gruca P, Lason-Tyburkiewicz M, Willner P (2017) Antidepressant, anxiolytic and procognitive effects of subacute and chronic ketamine in the chronic mild stress model of depression. Behav Pharmacol 28(1):1–8. https://doi.org/10.1097/FBP.0000000000000259
Foster CG, Landowski LM, Sutherland BA, Howells DW (2021) Differences in fatigue-like behavior in the lipopolysaccharide and poly I: C inflammatory animal models. Physiol Behav 232:113347. https://doi.org/10.1016/j.physbeh.2021.113347
Tracey KJ (2002) The inflammatory reflex. Nature 420(6917):853–859. https://doi.org/10.1038/nature01321
Njung’e K, Handley SL (1991) Evaluation of marble-burying behavior as a model of anxiety. Pharmacol Biochem Behav 38(1):63–67. https://doi.org/10.1016/0091-3057(91)90590-x
Broekkamp CL, Rijk HW, Joly-Gelouin D, Lloyd KL (1986) Major tranquillizers can be distinguished from minor tranquillizers on the basis of effects on marble burying and swim-induced grooming in mice. Eur J Pharmacol 126(3):223–229. https://doi.org/10.1016/0014-2999(86)90051-8
Deacon RM (2006) Digging and marble burying in mice: simple methods for in vivo identification of biological impacts. Nat Protoc 1(1):122–124. https://doi.org/10.1038/nprot.2006.20
Couch Y, Xie Q, Lundberg L, Sharp T, Anthony DC (2015) A model of post-infection fatigue is associated with increased TNF and 5-HT2A receptor expression in mice. PLoS ONE 10(7):e0130643. https://doi.org/10.1371/journal.pone.0130643
Vuillermot S, Luan W, Meyer U, Eyles D (2017) Vitamin D treatment during pregnancy prevents autism-related phenotypes in a mouse model of maternal immune activation. Mol Autism 7(8):9. https://doi.org/10.1186/s13229-017-0125-0
Manta S, Dong J, Debonnel G, Blier P (2009) Enhancement of the function of rat serotonin and norepinephrine neurons by sustained vagus nerve stimulation. J Psychiatry Neurosci 34(4):272–280
Acknowledgements
We are indebted to the former and present members of the Neurophysiology Laboratory at Daejeon University for their valuable discussions and comments. We are grateful to Eui Namgung (University of California, San Diego) for critically reading the manuscript.
Funding
This work was supported by the Basic Science Research Programs through the National Research Foundation of Korea (NRF) funded by the Ministry of Education (NRF-2018R1A6A1A03025221) and the Daejeon University fund (grant number 20220159).
Author information
Authors and Affiliations
Contributions
All experiments were performed in the Daejeon University. All authors contributed to the study conception and design. Material preparation, data collection and analysis were performed by Ki-Joong Kim, Jinyeon Hwang, Kang-Woo Lee, Jieun Kim, Yunha Han, and Uk Namgung. The first draft of the manuscript was written by Uk Namgung and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics Approval and Consent to Participate
All animal care procedures were approved by the Committee on Use of Live Animals for Teaching and Research at Daejeon University.
Competing Interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Kim, KJ., Hwang, J., Lee, KW. et al. Neuron-Microglia Interaction is Involved in Anti-inflammatory Response by Vagus Nerve Stimulation in the Prefrontal Cortex of Rats Injected with Polyinosinic:Polycytidylic Acid. Mol Neurobiol (2024). https://doi.org/10.1007/s12035-024-04054-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12035-024-04054-2