Abstract
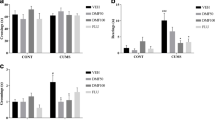
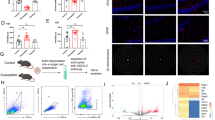
This work aimed to investigate the role of atractylenolide I (ATR) in resisting depression and its mechanism of action. The mouse model of depression was constructed through chronic unpredictable mild stress (CUMS) method. After ATR intervention, changes in the depression-related behaviors of mice were detected through open field test and elevated plus maze. In addition, enzyme-linked immunosorbent assay (ELISA) was conducted to detect inflammatory factor levels. Real-time fluorescence quantitative PCR (RT-qPCR) was performed to measure the mRNA levels of A1/A2 astrocyte markers. Furthermore, primary astrocytes were induced in vitro, and the A1 differentiation level was detected by ELISA and RT-qPCR assays. ATR improved the behaviors of CUMS mice and alleviated the depression symptoms. Moreover, it reduced tissue inflammation, inhibited the A1 differentiation of astrocytes, and decreased the mRNA levels of A1 markers. After NLRP3 knockout, the effects of ATR were suppressed. Similarly, in vitro experimental results also revealed that ATR suppressed the A1 differentiation of astrocytes. Based on molecular dynamics and small molecule-protein docking results, ATR mainly targeted NLRP3 and suppressed the NLRP3-mediated A1 differentiation. We discover that ATR can target NLRP3 to suppress A1 differentiation of astrocytes, restrain tissue inflammation, and improve the depression symptoms in mice.





Similar content being viewed by others
Data Availability
The data that support the findings of this study are available from the corresponding author upon reasonable request.
References
Sofroniew MV, Vinters HV (2010) Astrocytes: biology and pathology. Acta Neuropathol 119:7–35
Liddelow S, Barres B (2015) SnapShot: astrocytes in health and disease. Cell 162:1170-1170.e1171
Eng L, Vanderhaeghen J, Bignami A et al (1971) An acidic protein isolated from fibrous astrocytes. Brain Res 28:351–354
Hara M, Kobayakawa K, Ohkawa Y et al (2017) Interaction of reactive astrocytes with type I collagen induces astrocytic scar formation through the integrin-N-cadherin pathway after spinal cord injury. Nat Med 23:818–828
Myer DJ (2006) Essential protective roles of reactive astrocytes in traumatic brain injury. Brain 129:2761–2772
Liddelow SA, Barres BA (2017) Reactive astrocytes: production, function, and therapeutic potential. Immunity 46:957–967
Liddelow SA, Guttenplan KA, Clarke LE et al (2017) Neurotoxic reactive astrocytes are induced by activated microglia. Nature 541:481–487
Zamanian JL, Xu L, Foo LC et al (2012) Genomic analysis of reactive astrogliosis. J Neurosci 32:6391–6410
Hou B, Zhang Y, Liang P, He Y, Peng B, Liu W, Han S, Yin J et al (2020) Inhibition of the NLRP3-inflammasome prevents cognitive deficits in experimental autoimmune encephalomyelitis mice via the alteration of astrocyte phenotype. Cell Death Dis 11(5):377
Endo K, Taguchi T, Taguchi F et al (1979) Antiinflammatory principles of Atractylodes rhizomes. Chem Pharm Bull (Tokyo) 27(12):2954–2958
Kitajima J, Kamoshita A, Ishikawa T et al (2003) Glycosides of Atractylodes ovata. Chem Pharm Bull (Tokyo) 51(9):1106–1108
Cheng Y, Mai JY, Hou TL et al (2016) Antiviral activities of atractylon from Atractylodis Rhizoma. Mol Med Rep 14(4):3704–3710
Iamsaard S, Kietinun S, Sattayasai J, Bunluepuech K, Wu AT, Choowong-In P (2023) Prevention of seminal vesicle damage by Mucuna pruriens var. pruriens seed extract in chronic unpredictable mild stress mice. Pharm Biol. 61(1):89–99
Hammond TR, McEllin B, Morton PD et al (2015) Endothelin-B receptor activation in astrocytes regulates the rate of oligodendrocyte regeneration during remyelination. Cell Rep 13:2090–2097
Faulkner JR, Herrmann JE, Woo MJ et al (2004) Reactive astrocytes protect tissue and preserve function after spinal cord injury. J Neurosci 24:2143–2155
Bélanger M, Magistretti PJ (2009) The role of astroglia in neuroprotection. Dialogues Clin Neurosci 11:281–295
Zhong Z, Wen Z, Darnell JE Jr (1994) Stat3: a STAT family member activated by tyrosine phosphorylation in response to epidermal growth factor and interleukin-6. Science 264:95–98
Hong S, Song MR (2014) STAT3 but not STAT1 is required for astrocyte differentiation. PLoS ONE 9:e86851
Anderson MA, Burda JE, Ren Y et al (2016) Astrocyte scar formation aids central nervous system axon regeneration. Nature 532:195–200
Kettenmann H, Verkhratsky A (2008) Neuroglia: the 150 years after. Trends Neurosci 31:653–659
Okada S, Nakamura M, Katoh H et al (2006) Conditional ablation of Stat3 or Socs3 discloses a dual role for reactive astrocytes after spinal cord injury. Nature Med 12:829–834
Kowiański P, Lietzau G, Czuba E et al (2018) BDNF: a key factor with multipotent impact on brain signaling and synaptic plasticity. Cell Mol Neurobiol 38:579–593
Xu X, Zhang A, Zhu Y et al (2018) MFG-E8 reverses microglial-induced neurotoxic astrocyte (A1) via NF-κ B and PI3K-Akt pathways. J Cell Physiol 234:904–914
Fujita A, Yamaguchi H, Yamasaki R et al (2018) Connexin 30 deficiency attenuates A2 astrocyte responses and induces severe neurodegeneration in a 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine hydrochloride Parkinson’s disease animal model. J Neuroinflammation 15:227
Zhang L, Lei Z, Guo Z et al (2020) Development of neuroregenerative gene therapy to reverse glial scar tissue back to neuron-enriched tissue. Front Cell Neurosci 14:594170
Ma M, Li H, Wu J et al (2020) Roles of prokineticin 2 in subarachnoid hemorrhage-induced early brain injury via regulation of phenotype polarization in astrocytes. Mol Neurobiol 57:3744–3758
Liu LR, Liu JC, Bao JS et al (2020) Interaction of microglia and astrocytes in the neurovascular unit. Front Immunol 11:1024
Acknowledgements
I need to thank my colleagues and friends for their help and support in the research. Thanks for the support of the Science and Technology Planning Project of Jiaxing.
Funding
This study is funded by the Science and Technology Planning Project of Jiaxing [2023AZ11001].
Author information
Authors and Affiliations
Contributions
Liping Zhai and Yongjia Sheng: Responsible for the operation of animal experiments and cell experiments. Jin Wang and Xiaohong Zhou: Responsible for data analysis and chart production. Wenyan Li and Shasha Wu: Responsible for writing and revising articles. Yi Yang: Provide funding and guidance for project implementation.
Corresponding author
Ethics declarations
Ethical Approval and Consent to Participate
The study is approved by the Ethics Committee.
Consent for Publication
All authors approved to publish the article.
Competing Interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Zhai, L., Sheng, Y., Wang, J. et al. Atractylenolide I Suppresses A1 Astrocyte Activation to Improve Depression in Mice. Mol Neurobiol (2024). https://doi.org/10.1007/s12035-024-04025-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12035-024-04025-7