Abstract
The pathogenesis of schizophrenia begins in early neurodevelopment and leads to excitatory-inhibitory imbalance. It is therefore essential that preclinical models used to understand disease, select drug targets and evaluate novel therapeutics encompass similar neurochemical deficits. One approach to improved preclinical modelling incorporates dual-hit neurodevelopmental insults, like neonatal administration of phencyclidine (PCP, to disrupt development of glutamatergic circuitry) then post-weaning isolation (Iso, to mimic adolescent social stress). We recently showed that male Lister-hooded rats exposed to PCP-Iso exhibit reduced hippocampal expression of the GABA interneuron marker calbindin. The current study expanded on this by investigating changes to additional populations of GABAergic interneurons in frontal cortical and hippocampal tissue from the same animals (by immunohistochemistry) as well as levels of GABA itself (via ELISA). Because inflammatory changes are also implicated in schizophrenia, we performed additional immunohistochemical evaluations of Iba-1 positive microglia as well as ELISA analysis of IL-6 in the same brain regions. Single-hit isolation-reared and dual-hit PCP-Iso rats both showed reduced parvalbumin immunoreactivity in the prelimbic/infralimbic region of the frontal cortex. However, this was more widespread in PCP-Iso, extending to the medial/ventral and lateral/dorsolateral orbitofrontal cortices. Loss of GABAergic markers was accompanied by increased microglial activation in the medial/ventral orbitofrontal cortices of PCP-Iso, together with frontal cortical IL-6 elevations not seen following single-hit isolation rearing. These findings enhance the face validity of PCP-Iso, and we advocate the use of this preclinical model for future evaluation of novel therapeutics—especially those designed to normalise excitatory-inhibitory imbalance or reduce neuroinflammation.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Current antipsychotics address the positive symptoms of schizophrenia in approximately 70% of patients but have limited effect on cognitive or negative symptoms. As a result, this disorder remains one of the top 10% causes of disability worldwide, with an approximate cost of $281.6 billion in the USA alone during 2020 and an average lifetime cost of $3.8 million per patient [1]. There have been extensive efforts to develop improved treatments acting via a diverse array of pharmacological targets, but unfortunately, most of those that showed promising activity in preclinical tests failed to progress beyond phase III clinical trials. This high attrition reinforces the need for improved preclinical models, to further elucidate disease neurobiology, select plausible new targets for drug development and enable more predictive evaluation of novel therapeutics [2].
The pathogenesis of schizophrenia begins in early neurodevelopment and leads to lasting excitatory-inhibitory imbalance [3]. There is post-mortem evidence that this is due, at least in part, to disruption of certain populations of GABAergic interneurons in the prefrontal cortex (PFC) and hippocampus. For example, in schizophrenia, these regions contain lower levels of mRNA encoding parvalbumin and somatostatin [4,5,6,7,8], which are markers for respective interneuron subgroups that predominantly target either the cell body and initial axon segment, or dendritic shafts and spines of pyramidal neurons. This is also apparent at the protein level, with patient samples showing fewer parvalbumin- and somatostatin-immunoreactive neurons [5, 9, 10], or reduced intensity of parvalbumin immunostaining [11]. Neuroinflammation has been proposed as a potential mechanism underlying these changes, since positron emission tomography (PET) and post-mortem immunohistochemistry studies in schizophrenia detect increased activation of the brains immune cells, microglia [12]. There are reports of increased microglial cell density in studies examining the major histocompatibility complex class II antigen HLA-DR [13,14,15], as well as a shift towards a more activated amoeboid morphology of cells labelled for ionised calcium–binding adaptor molecule 1 (Iba-1) [16]. These are accompanied by elevated levels of cytokines, including interleukin-6 (IL-6) [15, 17]. Regardless of whether this inflammation is a cause or consequence of interneuron dysfunction, it is desirable that preclinical models for schizophrenia should feature similar GABAergic and inflammatory changes. This is a realistic expectation in genetic [18] and neurodevelopmental models [19] but cannot be achieved with simple acute pharmacological manipulations.
One approach to producing more comprehensive preclinical models involves ‘dual-hit’ combinations of established perinatal and peripubertal interventions. The two ‘hits’ are each chosen to mirror different aspects of the delayed symptom onset and multiple neurotransmitter involvement characteristic of complex neurodevelopmental disorders like schizophrenia [20]. For example, neonatal NMDA receptor antagonist administration (between postnatal days 7 and 11 when sensitivity to their pro-apoptotic effects peaks [21]) followed by post-weaning isolation rearing of gregarious rat pups induces a more robust phenotype than either manipulation alone [22,23,24,25]. Particular advantages of combining neonatal phencyclidine (PCP) with isolation rearing (PCP-Iso) are more extensive cognitive impairment across a broader array of domains (including spatial reference and fear-motivated associative memory [22, 25]), plus altered social interaction and concomitant ultrasonic vocalizations [26, 27] that appear more akin to negative symptomatology than the increased aggression seen with single-hit isolation rearing [28]. These are accompanied by downregulation of hippocampal genes involved in glutamate metabolism, dopaminergic neurotransmission, and GABA receptor signalling, as well as those encoding parvalbumin and glutamic acid decarboxylase 67 (GAD67) [29]. PCP-Iso also have reduced hippocampal expression of the calcium-binding protein calbindin [30], which is present in subsets of GABAergic interneurons throughout strata oriens, radiatum and lacunosum-moleculare (where our counts were obtained) as well as glutamatergic cells within stratum pyramidale. This appears consistent with reduced numbers or a disordered pattern of calbindin-positive cells in schizophrenia [31, 32], and may contribute to the apparent improved predictive validity of PCP-Iso compared to single-hit counterparts [30]. To provide further insight, the current study investigated changes to additional parvalbumin- and somatostatin-positive populations of GABAergic interneurons in sub-regions of the frontal cortex and hippocampus plus calbindin-positive cells in the frontal cortex (by immunohistochemistry). We performed additional immunohistochemical evaluation of Iba-1-positive microglia, as well as ELISA analysis of IL-6 and levels of GABA itself in bulk tissue from the same regions. Findings provide an important backdrop against which to interpret neurochemical substrates of the accompanying visual recognition memory deficits [30] in the same animals.
Methods
Animals and experimental design
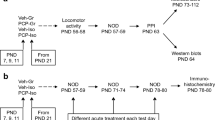
All procedures were conducted in accordance with the Animals (Scientific Procedures) Act, 1986, with approval from the University of Nottingham Animal Welfare and Ethical Review Body (AWERB). The research was designed and is reported in accordance with the Animal Research: Reporting of In Vivo Experiments (ARRIVE) guidelines [33]. It used stored brain tissue from a previously described cohort of 42 male Lister-hooded rats, in which the PCP-Iso group exhibited reduced hippocampal calbindin expression [30].
In summary, rats from a total of 6 litters were obtained with dams on postnatal day (PND) 3 (Charles River UK). They were maintained under controlled conditions throughout the study (21 ± 2 °C, 55 ± 10% humidity, 12-h light–dark cycle; on at 07:00 h) with unlimited access to food and water. Upon arrival, family groups were housed in individually ventilated cages (GR1800 Double-Decker; Tecniplast) containing sawdust bedding and standard environmental enrichment (cardboard play tube, wooden chew block and paper nest material). One pup died between delivery and the start of the study, and the remaining 41 pups, who each represented a single experimental unit, were randomised (by drawing lots) to receive neonatal administration of saline vehicle (Veh; 1 mL/kg s.c.) or PCP HCl (10 mg/kg base; Sigma-Aldrich) on PND 7, 9 and 11. Although this ensured each family group included a mix of both vehicle- and PCP-treated pups (which is an important design consideration to avoid any possibility of litter effects confounding the resulting data) it does introduce the possibility for cross-contamination (as a result of dams ingesting PCP during licking and grooming of drug-treated offspring and passing it to their suckling control offspring via the milk). It is reported that adult rats excrete 93% of an i.v. PCP dose via the urine and faeces within a week [34], but we do not have comparable data for neonatal animals following s.c. administration, nor any indication of milk levels in lactating females following p.o. administration. However, there were no more than three PCP-treated pups in any one litter, and their average cumulative body weights across the dosing period (128.1 ± 16.59 g) together with the pharmacokinetic information outlined above suggest it is possible that each dam had access to in the region of 1.19 mg of eliminated PCP over an 11 day period. Elimination of 93% of this by the routes outlined above leaves a maximum of 7% (0.0833 mg) available for incorporation into milk, and equal division between pups (6.83 per litter) results in a maximal possible cumulative p.o. dose of approximately 0.67 mg/kg to each individual. The dose ‘vehicle-treated’ pups might experience through this unavoidable cross-contamination therefore represents approximately 2% of that likely to encountered by PCP-treated pups through their combination of direct plus indirect routes, and the long-term consequences of this are likely to be minimal.
At weaning age (PND 21), the Veh-treated rats were further randomised (again by drawing lots) to rearing in standard groups of three or four per cage (Veh-Gr control; n = 14) or isolation, i.e. one per cage (single-hit Veh-Iso; n = 13). PCP-treated rats were all isolated (PCP-Iso dual hit; n = 14). Our study did not include a single-hit PCP-Gr condition because we have already shown that these animals do not exhibit cognitive dysfunction or lasting excitatory-inhibitory imbalance [25, 30]. Our focus here was to further understand the differences between Veh-Iso and PCP-Iso rather than their absence in PCP-Gr. The present approach allowed us to reduce total animal use by 25% and thereby comply with the reduction component of the 3Rs principle (replacement, reduction and refinement). Group sizes were based on previous studies employing the same techniques [24,25,26]. Following weaning, rats were housed in cages (Gr: 32 × 51 cm, Iso: 25 × 42 cm) containing sawdust bedding without environmental enrichment, and which had grid lids to ensure maintenance of visual, olfactory, and auditory contact between isolation-reared rats and other group- and singly housed rats within the same holding room [35]. Handling was restricted to a single weekly cage change and body weight measurement until behavioural testing.
To assess differences in pharmacological reversal of novel object discrimination (NOD) deficits between Veh-Iso and PCP-Iso, rats underwent NOD (as described in detail elsewhere [30]) on three separate occasions at 1–2 week intervals (PND 57–80). They received an acute i.p. administration of 0.5% methylcellulose 1% Tween-80 vehicle (1 mL/kg), the 5-HT6 receptor antagonist SB-399885 (10 mg/kg; Sigma-Aldrich) or mGlu7 antagonist MMPIP (10 mg/kg; Tocris), on separate test days, using a cross-over design and in a pseudorandom order. The humane endpoint would have been euthanasia of any rat experiencing a decrease in body weight (up to a maximum permitted limit of − 20%) and/or signs of poor body condition (e.g. piloerection, hunched posture, absence of grooming) although in practice none of these were encountered. Rats were killed by concussion and immediate decapitation on PND 79–80, straight after the final NOD test. The frontal cortex and hippocampus from one hemisphere were dissected on a refrigerated table (4 °C), weighed, frozen in liquid nitrogen and stored at − 80 °C for use in ELISAs. The remaining intact hemisphere was immerse fixed in 4% paraformaldehyde and cryopreserved in 30% sucrose (each overnight at 4 °C) then frozen in isopentane on dry ice and stored at − 80 °C for use in immunohistochemistry. Full blinding of experimenters to neurodevelopmental history throughout the 8–9 weeks of post-weaning housing was not possible due to the obvious visual difference between group and single housing. However, these allocations were concealed throughout tissue processing and analysis.
Immunohistochemical analysis of parvalbumin, somatostatin, calbindin and Iba-1
Serial coronal sections (60 μm) were obtained throughout the frontal cortex (Bregma 5.20 to 4.00) and dorsal hippocampus (Bregma − 2.56 to − 5.80 [36, 37]), using a freezing microtome (Anglia Scientific). They were stored in antifreeze (30% ethylene glycol; Fisher Scientific, and 30% glycerol; Honeywell, in 0.1 M phosphate buffered saline (PBS); Oxoid) at − 20 °C until free-floating immunohistochemistry. Tissue from one Veh-Gr and one Veh-Iso was excluded due to technical difficulties during slicing, resulting in final group sizes of n = 13 Veh-Gr, n = 12 Veh-Iso, and n = 14 PCP-Iso for immunohistochemistry.
For each brain region, six evenly spaced sections were processed for each of the four selected markers. Sections were washed (4 × 5 min) in PBS to remove antifreeze then incubated (1 h) in 2% normal goat serum (Abcam) in buffer 1 (0.5% bovine serum albumin (BSA); Sigma-Aldrich, 0.3% Triton-X100; Sigma-Aldrich, in PBS) to minimise non-specific binding of the secondary antibody to the tissue. Sections were incubated (overnight, 4 °C) in rabbit polyclonal antibodies against parvalbumin (Abcam ab11427, 1:1000), somatostatin (Abcam ab108456, 1:500), calbindin (Abcam ab108404, 1:500) or Iba-1 (Wako 019–19741, 1:2000), then washed (3 × 5 min) in buffer 2 (0.15% BSA and 0.1% Triton-X100 in PBS) to prevent any unbound primary antibody from interacting with the goat anti-rabbit Alexa-Fluor 568 secondary antibody (Abcam ab175471, 1:500; 1 h in the dark). A series of negative control sections were incubated in primary antibody alone, secondary antibody alone, or buffers only. Sections were washed (2 × 5 min each in buffer 2 then PBS), mounted on gelatinised slides and air-dried. Slides were rinsed with PBS, counterstained with DAPI nuclear stain (Sigma-Aldrich, 1:2000 in dH2O; 30 s) rinsed twice with dH2O and cover slipped with DABCO fluorescent mounting medium (Sigma-Aldrich; 0.2% in 90% glycerol in PBS) then stored at 4 °C.
To enable qualitative examination of the morphology of immunoreactive cells and subcellular localization of the signal, a small number of control sections were viewed using a Zeiss 880 confocal microscope. Representative × 40 images were obtained using Zen Black software (Zeiss). For quantitative analysis, sections were viewed on a Nikon EFD-3 fluorescence microscope and consistently placed × 10 snapshot images obtained from the medial/ventral orbitofrontal (MO/VO), lateral/dorsolateral orbitofrontal (LO/DLO), and prelimbic/infralimbic (PrL/IL) cortices (Fig. 1a) as well as the CA1, CA2/3, and dentate gyrus (DG) subfields of the hippocampus (Fig. 1b) using a Spot Insight 5MP CM05 USB camera and Spot Advanced software (v5.6; Diagnostic Instruments Inc.). Anatomical boundaries were determined using the stereotaxic brain atlas of Paxinos and Watson and a digital hippocampal atlas [36, 37]. Numbers of parvalbumin-, somatostatin-, calbindin- or Iba-1-positive cells per image were automatically counted with Fiji (Windows 32-bit [38]) by customizing an established protocol [39] to reflect optimal detection settings for each marker. Because it is theoretically possible there might be a decrease in the extent of immunoreactivity per expressing cell without any decrease in the density of immunoreactive cells the intensity of immunoreactivity in each image was automatically determined using the Analyse > Color Histogram tool in Fiji and normalised by subtraction of background staining [40]. In addition, the morphology of each individual Iba-1-positive cell was manually classified to provide an index of activation state. Cells with a small soma and expansive thin processes (whose length > soma diameter) were categorised as resting. Cells with a larger soma and shortened thickened processes (whose length still > soma diameter) as well as those transitioning towards a rod-like shape (narrowed elongated soma with few planar processes) were all classed as activated. Cells with a dramatically enlarged soma and very short or absent processes (whose length ≤ soma diameter) were classed as amoeboid [41, 42]. Data for each rat and brain region were averaged across the six sections per marker, such that n represents the number of biological and not technical replicates.
Immunohistochemical staining for GABAergic and inflammatory markers. Consistently placed snapshot images were collected from a medial/ventral orbitofrontal (MO/VO), lateral/dorsolateral orbitofrontal (LO/DLO) and prelimbic/infralimbic (PrL/IL) regions of the frontal cortex as well as b CA1, CA2/3 and dentate gyrus (DG) subfields of the dorsal hippocampus [36, 37]. Patterns of parvalbumin, somatostatin, calbindin and Iba-1 immunoreactivity in c confocal microscopy images obtained from the MO/VO for qualitative insight into the morphology of labelled cells and subcellular localization of the signal, as well as d standard fluorescence microscopy images from the MO/VO typical of those used for quantitative analysis. To aid placement of these images within the figure only one quarter of each image is presented. For each marker, e features were detected by automated counting settings. Representative higher magnification images from the MO/VO show f reduced parvalbumin expression and g increased activation state of Iba-1-positive microglia in rats that received PCP on postnatal day (PND) 7, 9 and 11 and were housed in isolation from weaning on PND21 (PCP-Iso), compared to rats that received vehicle injections and were housed in groups (Veh-Gr) or isolation (Veh-Iso). Scale bars are 10 μm in c and 100 μm in d–g. Arrowheads indicate g examples of activated microglia with enlarged cell bodies and shortened thickened processes, or transitioning towards a rod-like morphology with narrowed elongated cell bodies and fewer planar processes [41, 42]. Iba-1, ionised calcium–binding adapter molecule 1
ELISA analysis of GABA and IL-6 levels
Frontal cortical and hippocampal samples were homogenised (4 °C) in a 100:1 mix of radioimmunoprecipitation assay (RIPA) buffer and protease inhibitor cocktail (both Sigma-Aldrich). Buffer was added at a ratio of 100μL per 10 mg of tissue and protein extraction achieved by sonication (5–10 s, Soniprep 150; MSE) then vertical disc rotation (1 h). Supernatants resulting from centrifugation (5 min, RCF 850; Eppendorf 5417R) were stored at − 80 °C until analysis. Tissue samples from all animals were included in ELISAs, resulting in final group sizes of n = 14 Veh-Gr, n = 13 Veh-Iso and n = 14 PCP-Iso.
Total protein content was determined by Lowry assay. Forty microliters of BSA standards (0–0.5 mg/mL) and samples (1:100 in dH2O) were transferred to a flat bottomed 96-well plate and incubated (10 min) with 40μL of working Lowry reagent (1 part 0.5% copper sulphate pentahydrate, 1 part 2.7% potassium/sodium tartrate and 10 parts sodium carbonate in 0.5 M sodium hydroxide). Folin–Ciocalteu reagent was diluted (1:1 with dH2O) and added (20μL) to each well before incubation (45 min, in the dark). Absorbance at 750 nm was read using a SpectraMax M2e plate reader (Molecular Devices) with SoftMap Pro 7.1.2 software.
GABA and IL-6 content was determined against standards using commercially available ELISA kits (Biorbyt orb567856 and orb315058) according to the manufacturer’s instructions. Absorbance at 450 nm was read as described above. GABA and IL-6 concentrations were normalised to total protein concentrations within the same sample to correct for any minor differences in the efficiency of protein extraction between samples.
Statistical analysis
No exclusion criteria were set and data from all animals were included in the analyses. These were planned before the study took place (although not formally registered) and performed using GraphPad Prism v9.4.1. Normality was assessed with D’Agostino-Pearson or Kolmogorov–Smirnov tests. Based on the outcome of these, intensity of immunoreactivity and ELISA data were analysed by two-way repeated measures ANOVA (with neurodevelopmental condition as a between-subjects factor and brain region or sub-region as a within-subjects factor). The Geisser-Greenhouse correction for unequal variance was applied, and ANOVAs were followed with Tukey’s multiple comparisons post hoc test. Cell density and Iba-1 morphology data were analysed by non-parametric Kruskal–Wallis tests (applied separately to each brain sub-region, with neurodevelopmental condition as the sole factor) followed by Dunn’s post hoc. Data analysed with parametric tests are presented as bar charts showing mean ± standard error of the mean (SEM) and those analysed with non-parametric tests as box and whisker plots showing median, interquartile range (IQR) and 95% confidence intervals (CI). P < 0.05 was considered statistically significant.
Results
Validation of immunohistochemical staining for GABAergic and inflammatory markers
The selected primary antibodies have been used to visualise parvalbumin [43], somatostatin [44], calbindin [45] and Iba-1 [46] immunoreactivity in rat and mouse brain, and immunostaining in this study is consistent with previously observed patterns in the rat frontal cortex and hippocampus [41, 47, 48]. Thus, parvalbumin, calbindin and Iba-1 immunoreactivity were present throughout nuclei, cell bodies and processes (Fig. 1c–d). Abundant labelling of cell bodies enabled automated counting of immunoreactive cells to be reliably performed (Fig. 1e). Somatostatin immunoreactivity was less intense overall and showed a comparative absence from intracellular regions of the cell body (Fig. 1c–d). As a result, the settings necessary to automatically count cell bodies frequently also detected portions of immunoreactive processes irrespective of whether or not these were from the same or different cells (Fig. 1e). Somatostatin-positive cell counts should therefore be interpreted with caution. We have confidence in remaining data, which is maximised by that fact that immunoreactivity was only observed in sections incubated with respective primary plus the secondary antibody and abolished by the absence of primary and/or secondary antibodies (data not shown).
Impact of combined neonatal PCP and isolation rearing on GABAergic markers
There was a main effect of neurodevelopmental condition on parvalbumin immunoreactivity throughout the frontal cortex (F(2,36) = 11.40, P = 0.0001). This was reduced in the PrL/IL of both single-hit Veh-Iso and dual-hit PCP-Iso (− 14 and − 19%; P < 0.05 versus Veh-Gr control, Fig. 2a). Parvalbumin-positive cell density in the PrL/IL showed a similar effect of neurodevelopmental condition (Kruskal–Wallis statistic = 9.178, P = 0.0102) and was also reduced in both Veh-Iso and PCP-Iso (− 56 and − 49%; P < 0.05, Fig. 2b). Of note, PCP-Iso showed additional decreases in both the intensity of parvalbumin immunoreactivity in the MO/VO (− 23%; P < 0.01) and LO/DLO (− 16%; P < 0.05), and in the density of parvalbumin-positive cells in the MO/VO (− 71%; P < 0.01), neither of which were significantly reduced in Veh-Iso (Figs. 1f and 2a–b).
Impact of combined neonatal PCP and isolation rearing on GABAergic markers in the frontal cortex. Mean ± SEM intensity of a parvalbumin, c somatostatin and e calbindin immunoreactivity, together with median, IQR (box) and 95% CI (whiskers) for counts of b parvalbumin-, d somatostatin- and f calbindin-positive cell densities, as well as g mean ± SEM levels of GABA itself in both frontal cortical and hippocampal homogenates from opposite hemispheres of the same animals. Male Lister-hooded rats received saline vehicle (1 mL/kg s.c.; Veh) or PCP (10 mg/kg) on postnatal day (PND) 7, 9 and 11 were housed in groups (Gr) or isolation (Iso) from weaning on PND 21. They underwent novel object discrimination (NOD) three separate times at 1–2 week intervals (PND 57–80) following acute i.p. administration of 0.5% methylcellulose 1% Tween-80 vehicle (1 mL/kg), SB-399885 (10 mg/kg) or MMPIP (10 mg/kg) on separate days using a cross-over design. Tissue was collected on PND 79–80 (n = 12–14 per neurodevelopmental condition) and immunohistochemical data were obtained from consistently placed regions of interest within medial/ventral orbitofrontal (MO/VO), lateral/dorsolateral orbitofrontal (LO/DLO) and prelimbic/infralimbic (PrL/IL) sub-regions. *P < 0.05; **P < 0.01 Veh-Iso and PCP-Iso versus Veh-Gr; #P < 0.05 PCP-Iso versus Veh-Iso (a, c, e, g two-way repeated measures ANOVA with Tukey’s or b, d, f Kruskal–Wallis test with Dunn’s post hoc)
There was no effect of neurodevelopmental condition on the intensity of frontal cortical somatostatin immunoreactivity (F(2,36) = 0.056, P = 0.9455; Fig. 2c), and although somatostatin-positive cell density in the LO/DLO was influenced by neurodevelopmental condition (Kruskal–Wallis statistic = 8.442, P = 0.0147) there was no decrease in Veh-Iso or PCP-Iso compared to Veh-Gr. However density in PCP-Iso was higher than in Veh-Iso (Fig. 2d). As noted above, these data should be interpreted with a degree of caution (see Fig. 1e). There were no differences in calbindin immunoreactivity or cell densities in the frontal cortex (Fig. 2e–f), nor in any of the interneuron markers within the hippocampus (data not shown). Levels of GABA were not significantly impacted by neurodevelopmental condition, although interestingly apparent trends within the frontal cortex (Fig. 2g) mirrored the pattern of reduced parvalbumin immunoreactivity across this region (Fig. 2a–b).
Impact of combined neonatal PCP and isolation rearing on inflammatory markers
There was no effect of neurodevelopmental condition on overall expression of Iba-1, either in terms of immunoreactivity or cell densities in the frontal cortex (Fig. 3a–b) or hippocampus (data not shown). However, when Iba-1-positive cells were classified according to their morphology to obtain an index of activation state, there was an effect of neurodevelopmental condition on the percentage of MO/VO cells in the resting state (Kruskal–Wallis statistic = 6.371, P = 0.0414), which tended to decrease in PCP-Iso compared to Veh-Gr (0.0797; data not shown). Neurodevelopmental condition also influenced the percentage of MO/VO cells in the early stages of the activated state (Kruskal–Wallis statistic = 13.06, P = 0.0015), which was higher in PCP-Iso than both Veh-Gr (1.8-fold; P < 0.01) and Veh-Iso (1.6-fold; P < 0.05; Figs. 1g and 3c). The percentage of cells in the later, amoeboid stage of the activated state was extremely low (medians ≤ 1%, IQRs 0–1%) and not influenced by neurodevelopmental condition (data not shown). Levels of the inflammatory cytokine IL-6 within the frontal cortex were also higher in PCP-Iso (but not Veh-Iso) than Veh-Gr (P < 0.05; Fig. 3d). There were no differences in inflammatory markers within the hippocampus.
Impact of combined neonatal PCP and isolation rearing on inflammatory markers in the frontal cortex. Mean ± SEM a intensity of Iba-1 immunoreactivity, together with median, IQR (box) and 95% CI (whiskers) for b Iba-1-positive cell densities and the c percentage of Iba-1-positive microglia with morphological indications of an activated state [41, 42], as well as d mean ± SEM levels of the cytokine IL-6 in both frontal cortical and hippocampal homogenates from opposite hemispheres of the same animals. Male Lister-hooded rats received saline vehicle (1 mL/kg s.c.; Veh) or PCP (10 mg/kg) on postnatal day (PND) 7, 9 and 11 were housed in groups (Gr) or isolation (Iso) from weaning on PND 21. They underwent novel object discrimination (NOD) three separate times at 1–2 week intervals (PND 57–80) following acute i.p. administration of 0.5% methylcellulose 1% Tween-80 vehicle (1 mL/kg), SB-399885 (10 mg/kg) or MMPIP (10 mg/kg) on separate days using a cross-over design. Tissue was collected on PND 79–80 (n = 12–14 per neurodevelopmental condition) and immunohistochemical data were obtained from consistently placed regions of interest within medial/ventral orbitofrontal (MO/VO), lateral/dorsolateral orbitofrontal (LO/DLO) and prelimbic/infralimbic (PrL/IL) sub-regions. *P < 0.05; **P < 0.01 PCP-Iso versus Veh-Gr; #P < 0.05 PCP-Iso versus Veh-Iso (a, d two-way repeated measures ANOVA with Tukey’s or b, c Kruskal–Wallis test with Dunn’s post hoc)
Discussion
Improved preclinical models are essential to enable more predictive evaluation of urgently needed novel therapeutics for negative and cognitive symptoms of schizophrenia. The dual-hit combination of neonatal PCP followed by insolation rearing is reported to produce a wider range of behavioural and neurochemical changes than single-hit isolation rearing, and alterations in PCP-Iso appear more akin to those in schizophrenia [22, 25,26,27, 30]. The current study is the first to show additional parvalbumin deficits, microglial activation and IL-6 elevation within the frontal cortex of PCP-Iso, and these findings further support improved face validity of the dual-hit PCP-Iso model.
Decreased frontal cortical or hippocampal expression of parvalbumin has been reported following a variety of protocols involving some form of social isolation as a single developmental manipulation. This includes work in highly gregarious rodents like rats, and also studies in mice—despite males of this species generally being more aggressive towards conspecifics [49]. However, the outcomes in these single-hit models are variable in terms of the affected brain region (e.g., [50, 51] versus [52, 53]). Moreover, they are frequently localised to an individual sub-region, like the PrL or IL as observed in our Veh-Iso [24, 54,55,56], or even to a specific cell layer within a sub-region [57]. This explains why they might not be detected during analysis of pooled sub-regions [58]. To the best of our knowledge, no studies have assessed the impact of either post-weaning isolation rearing or adulthood social isolation on frontal cortical or hippocampal somatostatin expression. Isolation-induced changes to other markers appear more variable. For example, decreased hippocampal calbindin in our Veh-Iso [30] is more localised than in isolation-reared females from the same strain [50]. The current absence of any frontal cortical change to this marker is in keeping with one prior report [24] but conflicts with mixed findings from another group, who have reported both decreases [59] and increases [60]. Similarly, we [40, 61] and others [62, 63] variously detect increases, decreases or no change to levels of cytokines like IL-6 in the frontal cortex of isolates. Taken together, these findings reinforce the need for more robust models featuring a wider and more reproducible spectrum of changes akin to those in complex psychiatric disorders like schizophrenia.
There is growing appreciation for the value of dual-hit neurodevelopmental models [2, 20]. Although not all combinations of relevant maternal, dietary or social risk factors have an additive effect on rodent behavioural or neurochemical endpoints [40, 64, 65], there is now a strong body of evidence that the consequences of post-weaning social isolation are exacerbated by prior blockade of NMDA receptors during the neonatal period [22, 24,25,26,27, 30, 52, 66]. Reduced frontal cortical parvalbumin expression in PCP-Iso mirrors a number of post-mortem findings from schizophrenia patients over the past 25 years. This applies to both the dorsolateral PFC [10, 11, 67, 68] (whose closest functional homolog in rodents includes the PrL [69], impacted by both Veh-Iso and PCP-Iso here) and the orbitofrontal cortex [9] (crucially affected only by PCP-Iso). Evidence for reduced hippocampal parvalbumin in schizophrenia is more sparse [5, 70], and negative reports for both regions [71,72,73] have led to the proposal of a ‘low GABA marker’ subgroup, comprising approximately 50% of the patient population [74]. There is also an inflammatory subgroup [15, 75, 76] and whilst additional research is needed to clarify the proportion of patients exhibiting low GABA markers [74] in conjunction with increased brain regional cytokines [17], we feel PCP-Iso have the potential to provide insights into disease neurobiology and treatment strategies for this combined subgroup. There is ongoing debate whether reduced expression of calcium binding proteins in schizophrenia represents a selective loss of these proteins from the interneurons, or a loss of the cells themselves. Reports of reduced total cortical neuron number and density [77,78,79] point towards cell loss, yet these are conflicted by reports of unaltered neuronal numbers or density [80, 81]. Similar conflicts exist for studies that have examined mRNA encoding general GABAergic markers like isoforms of the GABA synthesis enzyme GAD [81,82,83,84] versus the vesicular GABA transporter (vGAT) [7, 85,86,87]. However, a recent meta-analysis of proton magnetic resonance spectroscopy in patients points towards lower levels of GABA itself within the PFC [88] and trends within the current PCP-Iso data appear to mimic this. We feel this may be indicative of an interneuron dysfunction that goes beyond a selective absence of parvalbumin from otherwise normal cells. A reduction in calcium binding protein content is proposed to render cells less excitable, due to reduced ability to buffer Ca2+ transients and regulate repolarization. This in turn would disinhibit control over pyramidal cell output, with a similar outcome predicted upon loss of parvalbumin-positive cells [89]. However this view may represent an oversimplification since frontal cortical slices from mice subjected to two weeks of social isolation in the critical post-weaning period (PND 21-35) and then allowed to resocialize actually exhibit increased excitability of parvalbumin-positive interneurons in layer V of the PrL [90, 91].
Veh-Iso and PCP-Iso from this cohort both exhibited deficits in visual recognition memory in the NOD task [30], which means this robust phenotype has been reliably observed in each of the nine PCP-Iso cohorts now examined in our laboratory, despite only occurring in 70% of our previous Veh-Iso studies [30, 35]. Localised disruption of parvalbumin-, somatostain- and neuronal nitric oxide synthase (nNOS)–positive interneurons in the hippocampus is sufficient to impair NOD [92], but cognitive deficits in our Veh-Iso and PCP-Iso cannot be attributed to altered parvalbumin, somatostatin or inflammatory marker expression in this region, nor to somatostatin or calbindin alterations in the frontal cortex since no changes were detected. Neurochemical disturbances in PCP-Iso will extend beyond the molecules [29] and brain regions [66] studied here, so we cannot definitively state that frontal cortical parvalbumin, microglial and/or IL-6 alterations contribute to accompanying cognitive changes. The medial PFC (mPFC) and orbitofrontal cortex are actually more strongly implicated in working memory, reversal learning, attentional set shifting and social cognition [93]. Nevertheless, lesions of the PrL and IL profoundly impair performance in a food-motivated object-based working memory test [94], and NOD is attenuated by localised administration of the GABAA receptor agonist muscimol into the mPFC [95]. With regard to parvalbumin-positive neurons in this region, localised knockdown of the calcium-binding protein or selective inhibition of GABA release from these cells reduces spatial working memory, reversal learning [96] and social interaction [97]. And although transient optogenetic inhibition of these neurons does not appear to impact these cognitive processes [98, 99], optogenetic stimulation or chemogenetic activation of parvalbumin-positive cells in the PFC is able to overcome NMDA receptor antagonist–induced impairments in working memory and cognitive flexibility [100, 101]. Whilst these elegant approaches have not been used to examine the role of frontal cortical parvalbumin-positive neurons in NOD, it is intriguing to note that acute inflammation elevates recruitment of these neurons within the PrL during this task [102]. It is therefore conceivable that reduced parvalbumin expression could contribute to NOD impairments, particularly in the face of PCP-Iso–induced inflammation. To provide more definitive insight into the neurochemical substrates of cognitive and social deficits in PCP-Iso future work should examine whether these can be reversed by optogenetic or chemogenetic stimulation of parvalbumin-positive neurons, as demonstrated for aggressive behaviour in a single-hit social isolation model [54].
We acknowledge that some of our methodological choices may have impacted on the findings, and future work should also attend to these considerations. For example, rats were bred at a commercial establishment and shipped to our laboratories as neonates, arriving (PND 3) four days before the start of treatment (PND 7). It is recognised that transportation can have lasting effects on a wide array of physiological and behavioural parameters, including plasma corticosterone [103], and the age-critical nature of our PCP administration regime allows limited scope for an extended acclimatization period while these normalise. Although one potential alternative would be to breed animals in-house to avoid the need for transport, it is conceivable that stress levels at the time of PCP administration and the start of isolation rearing may interact with our intentional interventions and contribute to the final phenotype. Under those circumstances attempted refinements could have negative implications in terms of replication, and a dedicated study would be needed to assess the consequences of such methodological change. We also acknowledge that the current analysis was restricted to male rats, and this decision was originally taken because there are well-recognised biological sex differences in the human illness we seek to model, including worse premorbid functioning, an earlier age at symptom onset and greater severity of negative symptoms in males [104]. Although previous literature shows that cognitive impairment [105] and localised parvalbumin deficits do occur in single-hit isolation-reared female rats [50], emerging findings demonstrate that microglial activation is much more pronounced in isolation-reared male than female mice [106]. We are yet to examine female PCP-Iso but the current findings in male PCP-Iso, together with indications that perhiperal inflammatory biomarkers in patients vary according to biological sex [107] and a general move towards representation of both sexes in preclinical and clinical trials [108], make studies in female PCP-Iso increasingly timely. Lastly, we note that the brain regional samples analysed here were obtained from rats that each received one administration of the 5-HT6 receptor antagonist SB-399885, one administration of the mGlu7 antagonist MMPIP and one administration of vehicle control prior to cognitive testing in the 3 weeks leading up to tissue collection for immunohistochemistry. It would be highly unlikely for acute treatment to impact on the immunohistochemical data presented here or in our prior manuscript from the same study [30], and treatments were all administered in a pseudorandom order that was fully balanced across Veh-Gr, Veh-Iso and PCP-Iso groups to avoid any potential confounds based on recency.
Atypical antipsychotic agents are able to correct some, though by no means all of the disordered behaviours in male PCP-Iso. Acute administration of aripiprazole or cariprazine reversed locomotor hyperactivity and NOD impairments without marked effects on social interaction and fear memory [27]. Similarly chronic administration of clozapine reversed locomotor hyperactivity and enhanced social recognition memory without normalizing sensorimotor gating or reversal learning [109]. The anticonvulsant lamotrigine, which blocks voltage-gated sodium channels to reduce excitatory neurotransmission, also reversed locomotor hyperactivity and improved NOD without correcting sensorimotor gating [29]. These reports are broadly consistent with the moderate clinical efficacy of these compounds in schizophrenia (e.g. [110,111,112]), and the model also replicates negative clinical findings with 5-HT6 receptor antagonists [30, 113]. Given our new confirmation of inflammation in PCP-Iso an obvious next step in our reverse translational assessments of predictive validity would be to examine an anti-inflammatory agent, since these have also shown modest efficacy in patients [114, 115]. Another key priority, given the current GABAergic findings, is positive modulators of the Kv3.1/3.2 voltage-gated potassium channels on parvalbumin-positive interneurons. These compounds appear to normalise parvalbumin-positive cell counts in alternative models for schizophrenia [116] and have recently shown promising effects on gamma oscillations in patients [117]. In terms of forward translation, chronic administration of a glycine transporter (GlyT1) inhibitor is so far the only manipulation to normalise social interaction and aberrant patterns of prosocial ultrasonic vocalizations in PCP-Iso [26]. This class of compounds have subsequently been shown to restore inhibitory neurotransmission and normalise excitatory-inhibitory imbalance [118], and emerging clinical evidence suggests they have real potential for improved therapeutic effect [119]. Metabotropic glutamate receptor 7 (mGlu7) negative allosteric modulators are at an earlier stage in the drug development pipeline [120, 121], and our preliminary demonstration that they reverse NOD deficits in PCP-Iso [30] certainly suggests more in-depth assessment in this model is now warranted. In conclusion, the current demonstration of frontal cortical parvalbumin reduction, microglial activation and cytokine elevation enhance the face validity of our PCP-Iso model, and support its use to further elucidate disease neurobiology and select plausible new targets for drug development. Accumulating evidence supports the predictive validity of the PCP-Iso model and we advocate more widespread adoption of this valuable tool for preclinical evaluation of novel therapeutics for schizophrenia—especially those designed to normalise excitatory-inhibitory imbalance or reduce neuroinflammation.
Data availability
The data generated during this study are available from the corresponding author on reasonable request.
References
Velligan DI, Rao S (2023) The epidemiology and global burden of schizophrenia. J Clin Psychiatry 84:MS21078COM5. https://doi.org/10.4088/JCP.MS21078COM5
Spark DL, Fornito A, Langmead CJ, Stewart GD (2022) Beyond antipsychotics: a twenty-first century update for preclinical development of schizophrenia therapeutics. Transl Psychiatry 12:147. https://doi.org/10.1038/s41398-022-01904-2
Howes OD, Shatalina E (2022) Integrating the neurodevelopmental and dopamine hypotheses of schizophrenia and the role of cortical excitation-inhibition balance. Biol Psychiatry 92:501–513. https://doi.org/10.1016/j.biopsych.2022.06.017
Mellios N, Huang HS, Baker SP, Galdzicka M, Ginns E, Akbarian S (2009) Molecular determinants of dysregulated GABAergic gene expression in the prefrontal cortex of subjects with schizophrenia. Biol Psychiatry 65:1006–1014. https://doi.org/10.1016/j.biopsych.2008.11.019
Konradi C, Yang CK, Zimmerman EI, Lohmann KM, Gresch P, Pantazopoulos H, Berretta S, Heckers S (2011) Hippocampal interneurons are abnormal in schizophrenia. Schizophr Res 131:165–173. https://doi.org/10.1016/j.schres.2011.06.007
Fung SJ, Fillman SG, Webster MJ, Shannon Weickert C (2014) Schizophrenia and bipolar disorder show both common and distinct changes in cortical interneuron markers. Schizophr Res 155:26–30. https://doi.org/10.1016/j.schres.2014.02.021
Hoftman GD, Volk DW, Bazmi HH, Li S, Sampson AR, Lewis DA (2015) Altered cortical expression of GABA-related genes in schizophrenia: illness progression vs developmental disturbance. Schizophr Bull 41:180–191. https://doi.org/10.1093/schbul/sbt178
Chung DW, Volk DW, Arion D, Zhang Y, Sampson AR, Lewis DA (2016) Dysregulated ErbB4 splicing in schizophrenia: selective effects on parvalbumin expression. Am J Psychiatry 173:60–68. https://doi.org/10.1176/appi.ajp.2015.15020150
Beasley CL, Reynolds GP (1997) Parvalbumin-immunoreactive neurons are reduced in the prefrontal cortex of schizophrenics. Schizophr Res 24:349–355. https://doi.org/10.1016/S0920-9964(96)00122-3
Glausier JR, Fish KN, Lewis DA (2014) Altered parvalbumin basket cell inputs in the dorsolateral prefrontal cortex of schizophrenia subjects. Mol Psychiatry 19:30–36. https://doi.org/10.1038/mp.2013.152
Enwright JF, Sanapala S, Foglio A, Berry R, Fish KN, Lewis DA (2016) Reduced labeling of parvalbumin neurons and perineuronal nets in the dorsolateral prefrontal cortex of subjects with schizophrenia. Neuropsychopharmacology 41:2206–2214. https://doi.org/10.1038/npp.2016.24
Laskaris LE, Di Biase MA, Everall I, Chana G, Christopoulos A, Skafidas E, Cropley VL, Pantelis C (2016) Microglial activation and progressive brain changes in schizophrenia. Br J Pharmacol 173:666–680. https://doi.org/10.1111/bph.13364
Radewicz K, Garey LJ, Gentleman SM, Reynolds R (2000) Increase in HLA-DR immunoreactive microglia in frontal and temporal cortex of chronic schizophrenics. J Neuropathol Exp Neurol 59:137–150. https://doi.org/10.1093/jnen/59.2.137
Wierzba-Bobrowicz T, Lewandowska E, Lechowicz W, Stepień T, Pasennik E (2005) Quantitative analysis of activated microglia, ramified and damage of processes in the frontal and temporal lobes of chronic schizophrenics. Folia Neuropathol 43:81–89
Fillman SG, Cloonan N, Catts VS, Miller LC, Wong J, McCrossin T, Cairns M, Weickert CS (2013) Increased inflammatory markers identified in the dorsolateral prefrontal cortex of individuals with schizophrenia. Mol Psychiatry 18:206–214. https://doi.org/10.1038/mp.2012.110
Hercher C, Chopra V, Beasley CL (2014) Evidence for morphological alterations in prefrontal white matter glia in schizophrenia and bipolar disorder. J Psychiatry Neurosci 39:376–385. https://doi.org/10.1503/jpn.130277
Pandey GN, Rizavi HS, Zhang H, Ren X (2018) Abnormal gene and protein expression of inflammatory cytokines in the postmortem brain of schizophrenia patients. Schizophr Res 192:247–254. https://doi.org/10.1016/j.schres.2017.04.043
Takao K, Kobayashi K, Hagihara H, Ohira K, Shoji H, Hattori S, Koshimizu H, Umemori J, Toyama K, Nakamura HK, Kuroiwa M, Maeda J, Atsuzawa K, Esaki K, Yamaguchi S, Furuya S, Takagi T, Walton NM, Hayashi N, Suzuki H, Higuchi M, Usuda N, Suhara T, Nishi A, Matsumoto M, Ishii S, Miyakawa T (2013) Deficiency of schnurri-2, an MHC enhancer binding protein, induces mild chronic inflammation in the brain and confers molecular, neuronal, and behavioral phenotypes related to schizophrenia. Neuropsychopharmacology 38:1409–1425. https://doi.org/10.1038/npp.2013.38
Ducharme G, Lowe GC, Goutagny R, Williams S (2012) Early alterations in hippocampal circuitry and theta rhythm generation in a mouse model of prenatal infection: implications for schizophrenia. PLoS ONE 7:e29754. https://doi.org/10.1371/journal.pone.0029754
Möller M, Swanepoel T, Harvey BH (2015) Neurodevelopmental animal models reveal the convergent role of neurotransmitter systems, inflammation, and oxidative stress as biomarkers of schizophrenia: implications for novel drug development. ACS Chem Neurosci 6:987–1016. https://doi.org/10.1021/cn5003368
Ikonomidou C, Bosch F, Miksa M, Bittigau P, Vöckler J, Dikranian K, Tenkova TI, Stefovska V, Turski L, Olney JW (1999) Blockade of NMDA receptors and apoptotic neurodegeneration in the developing brain. Science 283:70–74. https://doi.org/10.1126/science.283.5398.70
Gaskin PL, Alexander SP, Fone KC (2011) Combining rearing in social isolation with perinatal PCP treatment as a preclinical model of schizophrenia. J Psychopharmacol 25S:A70
Lim AL, Taylor DA, Malone DT (2012) A two-hit model: behavioural investigation of the effect of combined neonatal MK-801 administration and isolation rearing in the rat. J Psychopharmacol 26:1252–1264. https://doi.org/10.1177/0269881111430751
Gilabert-Juan J, Belles M, Saez AR, Carceller H, Zamarbide-Fores S, Moltó MD, Nacher J (2013) A “double hit” murine model for schizophrenia shows alterations in the structure and neurochemistry of the medial prefrontal cortex and the hippocampus. Neurobiol Dis 59:126–140. https://doi.org/10.1016/j.nbd.2013.07.008
Gaskin PL, Alexander SP, Fone KC (2014) Neonatal phencyclidine administration and post-weaning social isolation as a dual-hit model of “schizophrenia-like” behaviour in the rat. Psychopharmacology 231:2533–2545. https://doi.org/10.1007/s00213-013-3424-y
Kohli S, Alberati D, Ballard TM, Steward LJ, King MV, Fone KCF (2016) The GlyT1 inhibitor RO4993850 alters social behavior and ultrasonic vocalization calls in a neonatal-phencyclidine isolation-reared rat model for schizophrenia. Eur Neuropsychopharmacol 26:S494–S495. Also available online at https://www.ecnp.eu/presentationpdfs/70/P.3.a.012.pdf. Accessed 19 May 2023
Watson DJG, King MV, Gyertyán I, Kiss B, Adham N, Fone KCF (2016) The dopamine D3-preferring D2/D3 dopamine receptor partial agonist, cariprazine, reverses behavioural changes in a rat neurodevelopmental model for schizophrenia. Eur Neuropsychopharmacol 26:208–224. https://doi.org/10.1016/j.euroneuro.2015.12.020
Jones CA, Watson DJ, Fone KC (2011) Animal models of schizophrenia. Br J Pharmacol 164:1162–1194. https://doi.org/10.1111/j.1476-5381.2011.01386.x
Gaskin PL, Toledo-Rodriguez M, Alexander SP, Fone KC (2016) Down-regulation of hippocampal genes regulating dopaminergic, GABAergic, and glutamatergic function following combined neonatal phencyclidine and post-weaning social isolation of rats as a neurodevelopmental model for schizophrenia. Int J Neuropsychopharmacol 19:pyw062. https://doi.org/10.1093/ijnp/pyw062
Shortall SE, Brown AM, Newton-Mann E, Dawe-Lane E, Evans C, Fowler M, King MV (2020) Calbindin deficits may underlie dissociable effects of 5-HT6 and mGlu7 antagonists on glutamate and cognition in a dual-hit neurodevelopmental model for schizophrenia. Mol Neurobiol 57:3439–3457. https://doi.org/10.1007/s12035-020-01938-x
Iritani S, Kuroki N, Ikeda K, Kazamatsuri H (1999) Calbindin immunoreactivity in the hippocampal formation and neocortex of schizophrenics. Prog Neuropsychopharmacol Biol Psychiatry 23:409–421. https://doi.org/10.1016/S0278-5846(99)00005-6
Altar CA, Jurata LW, Charles V, Lemire A, Liu P, Bukhman Y, Young TA, Bullard J, Yokoe H, Webster MJ, Knable MB, Brockman JA (2005) Deficient hippocampal neuron expression of proteasome, ubiquitin, and mitochondrial genes in multiple schizophrenia cohorts. Biol Psychiatry 58:85–96. https://doi.org/10.1016/j.biopsych.2005.03.031
Percie du Sert N, Hurst V, Ahluwalia A, Alam S, Avey MT, Baker M, Browne WJ, Clark A, Cuthill IC, Dirnagl U, Emerson M, Garner P, Holgate ST, Howells DW, Karp NA, Lazic SE, Lidster K, MacCallum CJ, Macleod M, Pearl EJ, Petersen OH, Rawle F, Reynolds P, Rooney K, Sena ES, Silberberg SD, Steckler T, Würbel H (2020) The ARRIVE guidelines 2.0: updated guidelines for reporting animal research. Br J Pharmacol 177:3617–3624. https://doi.org/10.1111/bph.15193
Chakrabarti S, Law FC (1983) The dispositional kinetics of phencyclidine and its N-ethylamine analogue in rats. Eur J Drug Metab Pharmacokinet 8:383–388. https://doi.org/10.1007/BF03188770
Fone KC, Porkess MV (2008) Behavioural and neurochemical effects of post-weaning social isolation in rodents-relevance to developmental neuropsychiatric disorders. Neurosci Biobehav Rev 32:1087–1102. https://doi.org/10.1016/j.neubiorev.2008.03.003
Paxinos G, Watson C (2006) The rat brain in stereotaxic coordinates, 6th edn. Academic Press; Cambridge, MA
Kjonigsen LJ, Leergaard TB, Witter MP, Bjaalie JG (2011) Digital atlas of anatomical subdivisions and boundaries of the rat hippocampal region. Front Neuroinform 5:2. https://doi.org/10.3389/fninf.2011.00002
Schindelin J, Arganda-Carreras I, Frise E, Kaynig V, Longair M, Pietzsch T, Preibisch S, Rueden C, Saalfeld S, Schmid B, Tinevez JY, White DJ, Hartenstein V, Eliceiri K, Tomancak P, Cardona A (2012) Fiji: an open-source platform for biological-image analysis. Nat Methods 9:676–682. https://doi.org/10.1038/nmeth.2019
Labno C (2014) Two ways to count cells with ImageJ. https://www.unige.ch/medecine/bioimaging/files/3714/1208/5964/CellCounting.pdf. Accessed 7 March 2022
Goh JY, O’Sullivan SE, Shortall SE, Zordan N, Piccinini AM, Potter HG, Fone KCF, King MV (2020) Gestational poly(I:C) attenuates, not exacerbates, the behavioral, cytokine and mTOR changes caused by isolation rearing in a rat “dual-hit” model for neurodevelopmental disorders. Brain Behav Immun 89:100–117. https://doi.org/10.1016/j.bbi.2020.05.076
Cotel MC, Lenartowicz EM, Natesan S, Modo MM, Cooper JD, Williams SC, Kapur S, Vernon AC (2015) Microglial activation in the rat brain following chronic antipsychotic treatment at clinically relevant doses. Eur Neuropsychopharmacol 25:2098–2107. https://doi.org/10.1016/j.euroneuro.2015.08.004
Taylor SE, Morganti-Kossmann C, Lifshitz J, Ziebell JM (2014) Rod microglia: a morphological definition. PLoS ONE 9:e97096. https://doi.org/10.1371/journal.pone.0097096
Reichelt AC, Lemieux CA, Princz-Lebel O, Singh A, Bussey TJ, Saksida LM (2021) Age-dependent and region-specific alteration of parvalbumin neurons, perineuronal nets and microglia in the mouse prefrontal cortex and hippocampus following obesogenic diet consumption. Sci Rep 11:5593. https://doi.org/10.1038/s41598-021-85092-x
Espinosa N, Alonso A, Morales C, Espinosa P, Chávez AE, Fuentealba P (2019) Basal forebrain gating by somatostatin neurons drives prefrontal cortical activity. Cereb Cortex 29:42–53. https://doi.org/10.1093/cercor/bhx302
Nuryyev RL, Uhlendorf TL, Tierney W, Zatikyan S, Kopyov O, Kopyov A, Ochoa J, Van Trigt W, Malone CS, Cohen RW (2017) Transplantation of human neural progenitor cells reveals structural and functional improvements in the spastic Han-Wistar rat model of ataxia. Cell Transplant 26:1811–1821. https://doi.org/10.1177/096368971772363
Blaszczyk L, Maître M, Lesté-Lasserre T, Clark S, Cota D, Oliet SHR, Fénelon VS (2018) Sequential alteration of microglia and astrocytes in the rat thalamus following spinal nerve ligation. J Neuroinflammation 15:349. https://doi.org/10.1186/s12974-018-1378-z
Kubota Y, Hattori R, Yui Y (1994) Three distinct subpopulations of GABAergic neurons in rat frontal agranular cortex. Brain Res 649:159–273. https://doi.org/10.1016/0006-8993(94)91060-X
Nomura T, Fukuda T, Aika Y, Heizmann CW, Emson PC, Kobayashi T, Kosaka T (1997) Distribution of nonprincipal neurons in the rat hippocampus, with special reference to their dorsoventral difference. Brain Res 751:64–80. https://doi.org/10.1016/S0006-8993(96)01395-9
Lidster K, Owen K, Browne WJ, Prescott MJ (2019) Cage aggression in group-housed laboratory male mice: an international data crowdsourcing project. Sci Rep 9:15211. https://doi.org/10.1038/s41598-019-51674-z
Harte MK, Powell SB, Swerdlow NR, Geyer MA, Reynolds GP (2007) Deficits in parvalbumin and calbindin immunoreactive cells in the hippocampus of isolation reared rats. J Neural Transm (Vienna) 114:893–898. https://doi.org/10.1007/s00702-007-0627-6
Powell SB, Khan A, Young JW, Scott CN, Buell MR, Caldwell S, Tsan E, de Jong LA, Acheson DT, Lucero J, Geyer MA, Behrens MM (2015) Early adolescent emergence of reversal learning impairments in isolation-reared rats. Dev Neurosci 37:253–262. https://doi.org/10.1159/000430091
Schiavone S, Sorce S, Dubois-Dauphin M, Jaquet V, Colaianna M, Zotti M, Cuomo V, Trabace L, Krause KH (2009) Involvement of NOX2 in the development of behavioral and pathologic alterations in isolated rats. Biol Psychiatry 66:384–392. https://doi.org/10.1016/j.biopsych.2009.04.033
Klimczak P, Rizzo A, Castillo-Gómez E, Perez-Rando M, Gramuntell Y, Beltran M, Nacher J (2021) Parvalbumin interneurons and perineuronal nets in the hippocampus and retrosplenial cortex of adult male mice after early social isolation stress and perinatal NMDA receptor antagonist treatment. Front Synaptic Neurosci 13:733989. https://doi.org/10.3389/fnsyn.2021.733989
Li X, Sun H, Zhu Y, Wang F, Wang X, Han L, Cui D, Luo D, Zhai Y, Zhuo L, Xu X, Yang J, Li Y (2022) Dysregulation of prefrontal parvalbumin interneurons leads to adult aggression induced by social isolation stress during adolescence. Front Mol Neurosci 15:1010152. https://doi.org/10.3389/fnmol.2022.1010152
Todorović N, Mićić B, Schwirtlich M, Stevanović M, Filipović D (2019) Subregion-specific protective effects of fluoxetine and clozapine on parvalbumin expression in medial prefrontal cortex of chronically isolated rats. Neuroscience 396:24–35. https://doi.org/10.1016/j.neuroscience.2018.11.008
Ueno H, Suemitsu S, Murakami S, Kitamura N, Wani K, Okamoto M, Matsumoto Y, Ishihara T (2017) Region-specific impairments in parvalbumin interneurons in social isolation-reared mice. Neuroscience 359:196–208. https://doi.org/10.1016/j.neuroscience.2017.07.016
Perić I, Stanisavljević A, Gass P, Filipović D (2021) Fluoxetine exerts subregion/layer specific effects on parvalbumin/GAD67 protein expression in the dorsal hippocampus of male rats showing social isolation-induced depressive-like behaviour. Brain Res Bull 173:174–183. https://doi.org/10.1016/j.brainresbull.2021.05.021
Kaalund SS, Riise J, Broberg BV, Fabricius K, Karlsen AS, Secher T, Plath N, Pakkenberg B (2013) Differential expression of parvalbumin in neonatal phencyclidine-treated rats and socially isolated rats. J Neurochem 124:548–557. https://doi.org/10.1111/jnc.12061
Pascual R, Zamora-León P, Catalán-Ahumada M, Valero-Cabré A (2007) Early social isolation decreases the expression of calbindin D-28k and dendritic branching in the medial prefrontal cortex of the rat. Int J Neurosci 117:465–476. https://doi.org/10.1080/00207450600773459
Pascual R, Zamora-León P, Bustamante C (2013) Selegiline (deprenyl) decreases calbindin-D28k expression in cortical neurons of rats socially deprived during the post-weaning period. Int J Dev Neurosci 31:145–149. https://doi.org/10.1016/j.ijdevneu.2012.12.001
Dunphy-Doherty F, O’Mahony SM, Peterson VL, O’Sullivan O, Crispie F, Cotter PD, Wigmore P, King MV, Cryan JF, Fone KCF (2018) Post-weaning social isolation of rats leads to long-term disruption of the gut microbiota-immune-brain axis. Brain Behav Immun 68:261–273. https://doi.org/10.1016/j.bbi.2017.10.024
Perić I, Stanisavljević A, Gass P, Filipović D (2017) Fluoxetine reverses behavior changes in socially isolated rats: role of the hippocampal GSH-dependent defense system and proinflammatory cytokines. Eur Arch Psychiatry Clin Neurosci 267:737–749. https://doi.org/10.1007/s00406-017-0807-9
Corsi-Zuelli F, Fachim HA, Loureiro CM, Shuhama R, Bertozi G, Joca SRL, Menezes PR, Louzada-Junior P, Del-Ben CM (2019) Prolonged periods of social isolation from weaning reduce the anti-inflammatory cytokine IL-10 in blood and brain. Front Neurosci 12:1011. https://doi.org/10.3389/fnins.2018.01011
Ellenbroek BA, Cools AR (2002) Early maternal deprivation and prepulse inhibition: the role of the postdeprivation environment. Pharmacol Biochem Behav 73:177–184. https://doi.org/10.1016/S0091-3057(02)00794-3
Arcego DM, Krolow R, Lampert C, Toniazzo AP, Berlitz C, Lazzaretti C, Schmitz F, Rodrigues AF, Wyse AT, Dalmaz C (2016) Early life adversities or high fat diet intake reduce cognitive function and alter BDNF signaling in adult rats: interplay of these factors changes these effects. Int J Dev Neurosci 50:16–25. https://doi.org/10.1016/j.ijdevneu.2016.03.001
Castillo-Gómez E, Pérez-Rando M, Bellés M, Gilabert-Juan J, Llorens JV, Carceller H, Bueno-Fernández C, García-Mompó C, Ripoll-Martínez B, Curto Y, Sebastiá-Ortega N, Moltó MD, Sanjuan J, Nacher J (2017) Early social isolation stress and perinatal NMDA receptor antagonist treatment induce changes in the structure and neurochemistry of inhibitory neurons of the adult amygdala and prefrontal cortex. eNeuro 4:ENEURO.0034–17.2017. https://doi.org/10.1523/ENEURO.0034-17.2017
Lewis DA, Cruz DA, Melchitzky DS, Pierri JN (2001) Lamina-specific deficits in parvalbumin-immunoreactive varicosities in the prefrontal cortex of subjects with schizophrenia: evidence for fewer projections from the thalamus. Am J Psychiatry 158:1411–1422. https://doi.org/10.1176/appi.ajp.158.9.1411
Kaar SJ, Angelescu I, Marques TR, Howes OD (2019) Pre-frontal parvalbumin interneurons in schizophrenia: a meta-analysis of post-mortem studies. J Neural Transm (Vienna) 126:1637–1651. https://doi.org/10.1007/s00702-019-02080-2
Uylings HB, Groenewegen HJ, Kolb B (2003) Do rats have a prefrontal cortex? Behav Brain Res 146:3–17. https://doi.org/10.1016/j.bbr.2003.09.028
Knable MB, Barci BM, Webster MJ, Meador-Woodruff J, Torrey EF; Stanley Neuropathology Consortium (2004) Molecular abnormalities of the hippocampus in severe psychiatric illness: postmortem findings from the Stanley Neuropathology Consortium. Mol Psychiatry 9:609–620. https://doi.org/10.1038/sj.mp.4001471
Falkai P, Steiner J, Malchow B, Shariati J, Knaus A, Bernstein HG, Schneider-Axmann T, Kraus T, Hasan A, Bogerts B, Schmitt A (2016) Oligodendrocyte and interneuron density in hippocampal subfields in schizophrenia and association of oligodendrocyte number with cognitive deficits. Front Cell Neurosci 10:78. https://doi.org/10.3389/fncel.2016.00078
Woo TU, Miller JL, Lewis DA (1997) Schizophrenia and the parvalbumin-containing class of cortical local circuit neurons. Am J Psychiatry 154:1013–1015. https://doi.org/10.1176/ajp.154.7.1013
Tooney PA, Chahl LA (2004) Neurons expressing calcium-binding proteins in the prefrontal cortex in schizophrenia. Prog Neuropsychopharmacol Biol Psychiatry 28:273–278. https://doi.org/10.1016/j.pnpbp.2003.10.004
Volk DW, Sampson AR, Zhang Y, Edelson JR, Lewis DA (2016) Cortical GABA markers identify a molecular subtype of psychotic and bipolar disorders. Psychol Med 46:2501–2512. https://doi.org/10.1017/S0033291716001446
Goldsmith DR, Haroon E, Miller AH, Strauss GP, Buckley PF, Miller BJ (2018) TNF-α and IL-6 are associated with the deficit syndrome and negative symptoms in patients with chronic schizophrenia. Schizophr Res 199:281–284. https://doi.org/10.1016/j.schres.2018.02.048
Wang D, Wang Y, Chen Y, Yu L, Wu Z, Liu R, Ren J, Fang X, Zhang C (2023) Differences in inflammatory marker profiles and cognitive functioning between deficit and nondeficit schizophrenia. Front Immunol 13:958972. https://doi.org/10.3389/fimmu.2022.958972
Benes FM, McSparren J, Bird ED, SanGiovanni JP, Vincent SL (1991) Deficits in small interneurons in prefrontal and cingulate cortices of schizophrenic and schizoaffective patients. Arch Gen Psychiatry 48:996–1001. https://doi.org/10.1001/archpsyc.1991.01810350036005
Gaus R, Popal M, Heinsen H, Schmitt A, Falkai P, Hof PR, Schmitz C, Vollhardt A (2022) Reduced cortical neuron number and neuron density in schizophrenia with focus on area 24: a post-mortem case-control study. Eur Arch Psychiatry Clin Neurosci. https://doi.org/10.1007/s00406-022-01513-6
Thune JJ, Uylings HB, Pakkenberg B (2001) No deficit in total number of neurons in the prefrontal cortex in schizophrenics. J Psychiatr Res 35:15–21. https://doi.org/10.1016/S0022-3956(00)00043-1
Stark AK, Uylings HB, Sanz-Arigita E, Pakkenberg B (2004) Glial cell loss in the anterior cingulate cortex, a subregion of the prefrontal cortex, in subjects with schizophrenia. Am J Psychiatry 161:882–888. https://doi.org/10.1176/appi.ajp.161.5.882
Akbarian S, Kim JJ, Potkin SG, Hagman JO, Tafazzoli A, Bunney WE Jr, Jones EG (1995) Gene expression for glutamic acid decarboxylase is reduced without loss of neurons in prefrontal cortex of schizophrenics. Arch Gen Psychiatry 52:258–266. https://doi.org/10.1001/archpsyc.1995.03950160008002
Hashimoto T, Arion D, Unger T, Maldonado-Avilés JG, Morris HM, Volk DW, Mirnics K, Lewis DA (2008) Alterations in GABA-related transcriptome in the dorsolateral prefrontal cortex of subjects with schizophrenia. Mol Psychiatry 13:147–161. https://doi.org/10.1038/sj.mp.4002011
Woo TU, Kim AM, Viscidi E (2008) Disease-specific alterations in glutamatergic neurotransmission on inhibitory interneurons in the prefrontal cortex in schizophrenia. Brain Res 1218:267–277. https://doi.org/10.1016/j.brainres.2008.03.092
Thompson M, Weickert CS, Wyatt E, Webster MJ (2009) Decreased glutamic acid decarboxylase(67) mRNA expression in multiple brain areas of patients with schizophrenia and mood disorders. J Psychiatr Res 43:970–977. https://doi.org/10.1016/j.jpsychires.2009.02.005
Fung SJ, Sivagnanasundaram S, Weickert CS (2011) Lack of change in markers of presynaptic terminal abundance alongside subtle reductions in markers of presynaptic terminal plasticity in prefrontal cortex of schizophrenia patients. Biol Psychiatry 69:71–79. https://doi.org/10.1016/j.biopsych.2010.09.036
Rocco BR, Lewis DA, Fish KN (2016) Markedly lower glutamic acid decarboxylase 67 protein levels in a subset of boutons in schizophrenia. Biol Psychiatry 79:1006–1015. https://doi.org/10.1016/j.biopsych.2015.07.022
Dienel SJ, Fish KN, Lewis DA (2023) The nature of prefrontal cortical GABA neuron alterations in schizophrenia: markedly lower somatostatin and parvalbumin gene expression without missing neurons. Am J Psychiatry. https://doi.org/10.1176/appi.ajp.20220676
Simmonite M, Steeby CJ, Taylor SF (2023) Medial frontal cortex GABA concentrations in psychosis spectrum and mood disorders: a meta-analysis of proton magnetic resonance spectroscopy studies. Biol Psychiatry 93:125–136. https://doi.org/10.1016/j.biopsych.2022.08.004
Eyles DW, McGrath JJ, Reynolds GP (2002) Neuronal calcium-binding proteins and schizophrenia. Schizophr Res 57:27–34. https://doi.org/10.1016/s0920-9964(01)00299-7
Yamamuro K, Yoshino H, Ogawa Y, Okamura K, Nishihata Y, Makinodan M, Saito Y, Kishimoto T (2020) Juvenile social isolation enhances the activity of inhibitory neuronal circuits in the medial prefrontal cortex. Front Cell Neurosci 14:105. https://doi.org/10.3389/fncel.2020.00105
Okamura K, Yoshino H, Ogawa Y, Yamamuro K, Kimoto S, Yamaguchi Y, Nishihata Y, Ikehara M, Makinodan M, Saito Y, Kishimoto T (2023) Juvenile social isolation immediately affects the synaptic activity and firing property of fast-spiking parvalbumin-expressing interneuron subtype in mouse medial prefrontal cortex. Cereb Cortex 33:3591–3606. https://doi.org/10.1093/cercor/bhac294
Marks WD, Paris JJ, Schier CJ, Denton MD, Fitting S, McQuiston AR, Knapp PE, Hauser KF (2016) HIV-1 Tat causes cognitive deficits and selective loss of parvalbumin, somatostatin, and neuronal nitric oxide synthase expressing hippocampal CA1 interneuron subpopulations. J Neurovirol 22:747–762. https://doi.org/10.1007/s13365-016-0447-2
Ferguson BR, Gao WJ (2018) PV interneurons: critical regulators of E/I balance for prefrontal cortex-dependent behavior and psychiatric disorders. Front Neural Circuits 12:37. https://doi.org/10.3389/fncir.2018.00037
Kesner RP, Hunt ME, Williams JM, Long JM (1996) Prefrontal cortex and working memory for spatial response, spatial location, and visual object information in the rat. Cereb Cortex 6:311–318. https://doi.org/10.1093/cercor/6.2.311
de Landeta AB, Pereyra M, Miranda M, Bekinschtein P, Medina JH, Katche C (2021) Functional connectivity of anterior retrosplenial cortex in object recognition memory. Neurobiol Learn Mem 186:107544. https://doi.org/10.1016/j.nlm.2021.107544
Murray AJ, Woloszynowska-Fraser MU, Ansel-Bollepalli L, Cole KL, Foggetti A, Crouch B, Riedel G, Wulff P (2015) Parvalbumin-positive interneurons of the prefrontal cortex support working memory and cognitive flexibility. Sci Rep 5:16778. https://doi.org/10.1038/srep16778
Perez SM, Boley A, Lodge DJ (2019) Region specific knockdown of parvalbumin or somatostatin produces neuronal and behavioral deficits consistent with those observed in schizophrenia. Transl Psychiatry 9:264. https://doi.org/10.1038/s41398-019-0603-6
Abbas AI, Sundiang MJM, Henoch B, Morton MP, Bolkan SS, Park AJ, Harris AZ, Kellendonk C, Gordon JA (2018) Somatostatin interneurons facilitate hippocampal-prefrontal synchrony and prefrontal spatial encoding. Neuron 100:926–939. https://doi.org/10.1016/j.neuron.2018.09.029
Toader O, von Heimendahl M, Schuelert N, Nissen W, Rosenbrock H (2020) Suppression of parvalbumin interneuron activity in the prefrontal cortex recapitulates features of impaired excitatory/inhibitory balance and sensory processing in schizophrenia. Schizophr Bull 46:981–989. https://doi.org/10.1093/schbul/sbz123
Patrono E, Hrůzova K, Svoboda J, Stuchlík A (2023) The role of optogenetic stimulations of parvalbumin-positive interneurons in the prefrontal cortex and the ventral hippocampus on an acute MK-801 model of schizophrenia-like cognitive inflexibility. Schizophr Res 252:198–205. https://doi.org/10.1016/j.schres.2022.12.047
Chamberlin LA, Yang SS, McEachern EP, Lucas JTM, McLeod Ii OW, Rolland CA, Mack NR, Ferguson BR, Gao WJ (2023) Pharmacogenetic activation of parvalbumin interneurons in the prefrontal cortex rescues cognitive deficits induced by adolescent MK801 administration. Neuropsychopharmacology. https://doi.org/10.1038/s41386-023-01576-6
Feng XY, Hu HD, Chen J, Long C, Yang L, Wang L (2021) Acute neuroinflammation increases excitability of prefrontal parvalbumin interneurons and their functional recruitment during novel object recognition. Brain Behav Immun 98:48–58. https://doi.org/10.1016/j.bbi.2021.08.216
Arts JW, Kramer K, Arndt SS, Ohl F (2012) The impact of transportation on physiological and behavioral parameters in Wistar rats: implications for acclimatization periods. ILAR J 53:E82–E98. https://doi.org/10.1093/ilar.53.1.82
Giordano GM, Bucci P, Mucci A, Pezzella P, Galderisi S (2021) Gender differences in clinical and psychosocial features among persons with schizophrenia: a mini review. Front Psychiatry 12:789179. https://doi.org/10.3389/fpsyt.2021.789179
McLean S, Grayson B, Harris M, Protheroe C, Woolley M, Neill J (2010) Isolation rearing impairs novel object recognition and attentional set shifting performance in female rats. J Psychopharmacol 24:57–63. https://doi.org/10.1177/0269881108093842
Ayilara GO, Owoyele BV (2023) Neuroinflammation and microglial expression in brains of social-isolation rearing model of schizophrenia. IBRO Neurosci Rep 15:31–41. https://doi.org/10.1016/j.ibneur.2023.05.010
Ermakov EA, Melamud MM, Boiko AS, Kamaeva DA, Ivanova SA, Nevinsky GA, Buneva VN (2023) Association of peripheral inflammatory biomarkers and growth factors levels with sex, therapy and other clinical factors in schizophrenia and patient stratification based on these data. Brain Sci 13:836. https://doi.org/10.3390/brainsci13050836
DuMont M, Agostinis A, Singh K, Swan E, Buttle Y, Tropea D (2023) Sex representation in neurodegenerative and psychiatric disorders' preclinical and clinical studies. Neurobiol Dis 184:106214. https://doi.org/10.1016/j.nbd.2023.106214
Hamieh AM, Babin D, Sablé E, Hernier AM, Castagné V (2021) Neonatal phencyclidine and social isolation in the rat: effects of clozapine on locomotor activity, social recognition, prepulse inhibition, and executive functions deficits. Psychopharmacology 238:517–528. https://doi.org/10.1007/s00213-020-05700-y
Ribeiro ELA, de Mendonça LT, Vieira MEB, Storpirtis S, Aguiar PM (2018) Efficacy and safety of aripiprazole for the treatment of schizophrenia: an overview of systematic reviews. Eur J Clin Pharmacol 74:1215–1233. https://doi.org/10.1007/s00228-018-2498-1
Marder S, Fleischhacker WW, Earley W, Lu K, Zhong Y, Németh G, Laszlovszky I, Szalai E, Durgam S (2019) Efficacy of cariprazine across symptom domains in patients with acute exacerbation of schizophrenia: pooled analyses from 3 phase II/III studies. Eur Neuropsychopharmacol 29:127–136. https://doi.org/10.1016/j.euroneuro.2018.10.008
Puranen A, Koponen M, Lähteenvuo M, Tanskanen A, Tiihonen J, Taipale H (2023) Real-world effectiveness of mood stabilizer use in schizophrenia. Acta Psychiatr Scand 147:257–266. https://doi.org/10.1111/acps.13498
Morozova M, Burminskiy D, Rupchev G, Lepilkina T, Potanin S, Beniashvili A, Lavrovsky Y, Vostokova N, Ivaschenko A (2017) 5-HT6 receptor antagonist as an adjunct treatment targeting residual symptoms in patients with schizophrenia: unexpected sex-related effects (double-blind placebo-controlled trial). J Clin Psychopharmacol 37:169–175. https://doi.org/10.1097/JCP.0000000000000673
Jeppesen R, Christensen RHB, Pedersen EMJ, Nordentoft M, Hjorthøj C, Köhler-Forsberg O, Benros ME (2020) Efficacy and safety of anti-inflammatory agents in treatment of psychotic disorders - a comprehensive systematic review and meta-analysis. Brain Behav Immun 90:364–380. https://doi.org/10.1016/j.bbi.2020.08.028
Panizzutti B, Skvarc D, Lin S, Croce S, Meehan A, Bortolasci CC, Marx W, Walker AJ, Hasebe K, Kavanagh BE, Morris MJ, Mohebbi M, Turner A, Gray L, Berk L, Walder K, Berk M, Dean OM (2023) Minocycline as treatment for psychiatric and neurological conditions: a systematic review and meta-analysis. Int J Mol Sci 24:5250. https://doi.org/10.3390/ijms24065250
Leger M, Alvaro G, Large C, Harte M, Neill J (2015) AUT6, a novel Kv3 channel modulator, reverses cognitive and neurobiological dysfunction in a rat model of relevance to schizophrenia symptomatology. Eur Neuropsychopharmacol 25:S480. https://doi.org/10.1016/S0924-977X(15)30653-2
Kaar SJ, Nottage JF, Angelescu I, Marques TR, Howes OD (2023) Gamma oscillations and potassium channel modulation in schizophrenia: targeting GABAergic dysfunction. Clin EEG Neurosci. https://doi.org/10.1177/15500594221148643
Chen-Engerer HJ, Jaeger S, Bondarenko R, Sprengel R, Hengerer B, Rosenbrock H, Mack V, Schuelert N (2022) Increasing the excitatory drive rescues excitatory/inhibitory imbalance and mismatch negativity deficit caused by parvalbumin specific GluA1 deletion. Neuroscience 496:190–204. https://doi.org/10.1016/j.neuroscience.2022.06.027
Rosenbrock H, Desch M, Wunderlich G (2023) Development of the novel GlyT1 inhibitor, iclepertin (BI 425809), for the treatment of cognitive impairment associated with schizophrenia. Eur Arch Psychiatry Clin Neurosci. https://doi.org/10.1007/s00406-023-01576-z
Cieślik P, Woźniak M, Kaczorowska K, Brański P, Burnat G, Chocyk A, Bobula B, Gruca P, Litwa E, Pałucha-Poniewiera A, Wąsik A, Pilc A, Wierońska J (2018) Negative allosteric modulators of mGlu7 receptor as putative antipsychotic drugs. Front Mol Neurosci 11:316. https://doi.org/10.3389/fnmol.2018.00316
Kaczorowska K, Stankiewicz A, Bugno R, Paluchowska MH, Burnat G, Brański P, Cieślik P, Wierońska JM, Milik M, Nowak M, Przybyłowicz A, Kozioł A, Hogendorf A, Hogendorf AS, Kalinowska-Tłuścik J, Duszyńska B, Pilc A, Bojarski AJ (2023) Design and synthesis of new quinazolin-4-one derivatives with negative mGlu7 receptor modulation activity and antipsychotic-like properties. Int J Mol Sci 24:1981. https://doi.org/10.3390/ijms24031981
Acknowledgements
We thank the School of Life Sciences imaging facility (SLIM), particularly Ian Ward, for assistance with confocal microscopy.
Funding
This work was funded by the University of Nottingham.
Author information
Authors and Affiliations
Contributions
MVK designed and conceived the study, performed the in vivo procedures and ELISA, and conducted statistical analysis of resulting data. JAC designed and performed immunohistochemistry and associated analysis with assistance from EJC, JJC, ARF, SM, HCW and JZ, under the supervision of MVK. JAC wrote the first draft of the manuscript and all authors contributed to and have approved the final version.
Corresponding author
Ethics declarations
Ethical approval
All procedures were conducted in accordance with the Animals (Scientific Procedures) Act, 1986, with approval from the University of Nottingham Animal Welfare and Ethical Review Body (AWERB).
Consent to participate
Not applicable, as this research did not involve human subjects.
Consent for publication
Not applicable, as this research did not involve human subjects.
Competing interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Cale, J.A., Chauhan, E.J., Cleaver, J.J. et al. GABAergic and inflammatory changes in the frontal cortex following neonatal PCP plus isolation rearing, as a dual-hit neurodevelopmental model for schizophrenia. Mol Neurobiol (2024). https://doi.org/10.1007/s12035-024-03987-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12035-024-03987-y