Abstract
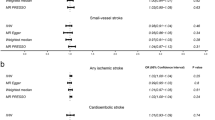
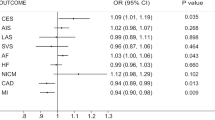
Cytokines and growth factors contribute to nerve growth and angiogenesis and are associated with the development of vascular disease. This Mendelian randomization (MR) study was designed to examine the causal relationship between factors associated with stem cell paracrine mechanisms and with stroke and its subtypes. We used pooled statistics on cytokine levels from three studies (INTERIAL, Olink Proseek CVD array, and KORA) encompassing 7795 participants in Europe. Data for stroke and its subtypes were pooled from these European populations (40,585 cases and 406,111 controls) in a multiprogenitor genome-wide association study (GWAS). MR was performed using established analytical methods, including inverse variance weighting (IVW), weighted median (WM), and MR-Egger. Genetically determined high IGF-1 levels were found to associate negatively with risk of stroke, ischemic stroke (large-artery atherosclerosis), and ischemic stroke (cardiogenic embolism). Meanwhile, high IL-13 levels had a positive causal relationship with ischemic stroke (large-artery atherosclerosis). An additional 27 cytokines were found to have a causal association with stroke or its subtypes. However, these results should be interpreted with caution given that the power efficacy was <80%. This MR study supports the concept of a causal relationship of 29 cytokines with stroke or its subtypes. Our genetic analysis provides new insights into stroke prevention and treatment by demonstrating an association of stem cell paracrine-related cytokines with stroke risk.







Similar content being viewed by others
Data Availability
All data generated or analyzed during this study are included in this article and its additional materials.
References
Collaborators GS (2021) Global, regional, and national burden of stroke and its risk factors, 1990-2019: a systematic analysis for the Global Burden of Disease Study 2019. Lancet Neurol 20(10):795–820. https://doi.org/10.1016/s1474-4422(21)00252-0
Esenwa CC, Elkind MS (2016) Inflammatory risk factors, biomarkers and associated therapy in ischaemic stroke. Nat Rev Neurol 12(10):594–604. https://doi.org/10.1038/nrneurol.2016.125
Schini-Kerth VB (1999) Dual effects of insulin-like growth factor-I on the constitutive and inducible nitric oxide (NO) synthase-dependent formation of NO in vascular cells. J Endocrinol Invest 22(5 Suppl):82–88
Ziv Y, Finkelstein A, Geffen Y et al (2007) A novel immune-based therapy for stroke induces neuroprotection and supports neurogenesis. Stroke 38(2 Suppl):774–782. https://doi.org/10.1161/01.Str.0000255784.27298.23
Kinnaird T, Stabile E, Burnett MS et al (2004) Marrow-derived stromal cells express genes encoding a broad spectrum of arteriogenic cytokines and promote in vitro and in vivo arteriogenesis through paracrine mechanisms. Circ Res 94(5):678–685. https://doi.org/10.1161/01.Res.0000118601.37875.Ac
Sze SK, de Kleijn DP, Lai RC et al (2007) Elucidating the secretion proteome of human embryonic stem cell-derived mesenchymal stem cells. Mol Cell Proteomics 6(10):1680–1689. https://doi.org/10.1074/mcp.M600393-MCP200
Sun BB, Maranville JC, Peters JE et al (2018) Genomic atlas of the human plasma proteome. Nature 558(7708):73–79. https://doi.org/10.1038/s41586-018-0175-2
Folkersen L, Fauman E, Sabater-Lleal M et al (2017) Mapping of 79 loci for 83 plasma protein biomarkers in cardiovascular disease. PLoS Genet 13(4):e1006706. https://doi.org/10.1371/journal.pgen.1006706
Suhre K, Arnold M, Bhagwat AM et al (2017) Connecting genetic risk to disease end points through the human blood plasma proteome. Nat Commun 8:14357. https://doi.org/10.1038/ncomms14357
Malik R, Chauhan G, Traylor M et al (2018) Multiancestry genome-wide association study of 520,000 subjects identifies 32 loci associated with stroke and stroke subtypes. Nat Genet 50(4):524–537. https://doi.org/10.1038/s41588-018-0058-3
Bowden J, Del Greco MF, Minelli C, Davey Smith G, Sheehan NA, Thompson JR (2016) Assessing the suitability of summary data for two-sample Mendelian randomization analyses using MR-Egger regression: the role of the I2 statistic. Int J Epidemiol 45(6):1961–1974. https://doi.org/10.1093/ije/dyw220
Pierce BL, Ahsan H, Vanderweele TJ (2011) Power and instrument strength requirements for Mendelian randomization studies using multiple genetic variants. Int J Epidemiol 40(3):740–752. https://doi.org/10.1093/ije/dyq151
Burgess S (2014) Sample size and power calculations in Mendelian randomization with a single instrumental variable and a binary outcome. Int J Epidemiol 43(3):922–929. https://doi.org/10.1093/ije/dyu005
Georgakis MK, Gill D, Rannikmäe K et al (2019) Genetically determined levels of circulating cytokines and risk of stroke. Circulation. 139(2):256–268. https://doi.org/10.1161/circulationaha.118.035905
Hutter R, Sauter BV, Reis ED et al (2003) Decreased reendothelialization and increased neointima formation with endostatin overexpression in a mouse model of arterial injury. Circulation 107(12):1658–1663. https://doi.org/10.1161/01.Cir.0000058169.21850.Ce
Spies M, Nesic O, Barrow RE, Perez-Polo JR, Herndon DN (2001) Liposomal IGF-1 gene transfer modulates pro- and anti-inflammatory cytokine mRNA expression in the burn wound. Gene Ther 8(18):1409–1415. https://doi.org/10.1038/sj.gt.3301543
Gillespie CM, Merkel AL, Martin AA (1997) Effects of insulin-like growth factor-I and LR3IGF-I on regional blood flow in normal rats. J Endocrinol 155(2):351–358. https://doi.org/10.1677/joe.0.1550351
Galderisi M, Caso P, Cicala S et al (2002) Positive association between circulating free insulin-like growth factor-1 levels and coronary flow reserve in arterial systemic hypertension. Am J Hypertens 15(9):766–772. https://doi.org/10.1016/s0895-7061(02)02967-9
Berryman DE, Glad CA, List EO, Johannsson G (2013) The GH/IGF-1 axis in obesity: pathophysiology and therapeutic considerations. Nat Rev Endocrinol 9(6):346–356. https://doi.org/10.1038/nrendo.2013.64
Perticone F, Sciacqua A, Tassone EJ et al (2012) One-hour post-load plasma glucose and IGF-1 in hypertensive patients. Eur J Clin Invest 42(12):1325–1331. https://doi.org/10.1111/eci.12005
Aguiar-Oliveira MH, Bartke A (2019) Growth hormone deficiency: health and longevity. Endocrine Rev. 40(2):575–601. https://doi.org/10.1210/er.2018-00216
Liang L, Wang J, Yuan Y et al (2018) MicRNA-320 facilitates the brain parenchyma injury via regulating IGF-1 during cerebral I/R injury in mice. Biomed. Pharmacother = Biomedecine & pharmacotherapie 102:86–93. https://doi.org/10.1016/j.biopha.2018.03.036
Saber H, Himali JJ, Beiser AS et al (2017) Serum insulin-like growth factor 1 and the risk of ischemic stroke: the Framingham Study. Stroke 48(7):1760–1765. https://doi.org/10.1161/strokeaha.116.016563
Chen D, Li J, Huang Y et al (2022) Interleukin 13 promotes long-term recovery after ischemic stroke by inhibiting the activation of STAT3. J Neuroinflammation 19(1):112. https://doi.org/10.1186/s12974-022-02471-5
Chung HS, Lee BS, Ma JY (2017) Ethanol extract of Mylabris phalerata inhibits M2 polarization induced by recombinant IL-4 and IL-13 in murine macrophages. Evid Based Complement Alternat Med 2017:4218468. https://doi.org/10.1155/2017/4218468
Hawkins KE, Corcelli M, Dowding K et al (2018) Embryonic stem cell-derived mesenchymal stem cells (MSCs) have a superior neuroprotective capacity over fetal MSCs in the hypoxic-ischemic mouse brain. Stem Cells Transl Med 7(5):439–449. https://doi.org/10.1002/sctm.17-0260
Kolosowska N, Keuters MH, Wojciechowski S et al (2019) Peripheral administration of IL-13 induces anti-inflammatory microglial/macrophage responses and provides neuroprotection in ischemic stroke. Neurotherapeutics 16(4):1304–1319. https://doi.org/10.1007/s13311-019-00761-0
Dinoro J, Maher M, Talebian S et al (2019) Sulfated polysaccharide-based scaffolds for orthopaedic tissue engineering. Biomaterials 214:119214. https://doi.org/10.1016/j.biomaterials.2019.05.025
Li X, Lin S, Chen X et al (2019) The prognostic value of serum cytokines in patients with acute ischemic stroke. Aging Dis 10(3):544–556. https://doi.org/10.14336/ad.2018.0820
Wada H, Suzuki M, Matsuda M et al (2020) Distinct characteristics of VEGF-D and VEGF-C to predict mortality in patients with suspected or known coronary artery disease. J Am Heart Assoc 9(9):e015761. https://doi.org/10.1161/jaha.119.015761
Berntsson J, Smith JG, Johnson LSB et al (2019) Increased vascular endothelial growth factor D is associated with atrial fibrillation and ischaemic stroke. Heart (British Cardiac Society) 105(7):553–558. https://doi.org/10.1136/heartjnl-2018-313684
Stiles CD, Capone GT, Scher CD, Antoniades HN, Van Wyk JJ, Pledger WJ (1979) Dual control of cell growth by somatomedins and platelet-derived growth factor. Proc Natl Acad Sci USA 76(3):1279–1283. https://doi.org/10.1073/pnas.76.3.1279
Narasimhalu K, Ma L, De Silva DA, Wong MC, Chang HM, Chen C (2015) Elevated platelet-derived growth factor AB/BB is associated with a lower risk of recurrent vascular events in stroke patients. Int J Stroke 10(1):85–89. https://doi.org/10.1111/ijs.12358
Marushima A, Nieminen M, Kremenetskaia I et al (2020) Balanced single-vector co-delivery of VEGF/PDGF-BB improves functional collateralization in chronic cerebral ischemia. J Cereb Blood Flow Metab 40(2):404–419. https://doi.org/10.1177/0271678x18818298
Zhang X, Bao L, Yang L, Wu Q, Li S (2012) Roles of intracellular fibroblast growth factors in neural development and functions. Sci China Life Sci 55(12):1038–1044. https://doi.org/10.1007/s11427-012-4412-x
Dordoe C, Chen K, Huang W et al (2021) Roles of fibroblast growth factors and their therapeutic potential in treatment of ischemic stroke. Front Pharmacol 12:671131. https://doi.org/10.3389/fphar.2021.671131
Li X, Wang C, Xiao J, McKeehan WL, Wang F (2016) Fibroblast growth factors, old kids on the new block. Semin Cell Dev Biol 53:155–167. https://doi.org/10.1016/j.semcdb.2015.12.014
Cheng X, Wang Z, Yang J et al (2011) Acidic fibroblast growth factor delivered intranasally induces neurogenesis and angiogenesis in rats after ischemic stroke. Neurol Res 33(7):675–680. https://doi.org/10.1179/1743132810y.0000000004
Tsai MJ, Tsai SK, Huang MC et al (2015) Acidic FGF promotes neurite outgrowth of cortical neurons and improves neuroprotective effect in a cerebral ischemic rat model. Neuroscience 305:238–247. https://doi.org/10.1016/j.neuroscience.2015.07.074
Wright CB, Dong C, Stark M et al (2014) Plasma FGF23 and the risk of stroke: the Northern Manhattan Study (NOMAS). Neurology 82(19):1700–1706. https://doi.org/10.1212/wnl.0000000000000410
Al Mamun A, Ngwa C, Qi S et al (2021) Neuronal CD200 signaling is protective in the acute phase of ischemic stroke. Stroke 52(10):3362–3373. https://doi.org/10.1161/strokeaha.120.032374
Zhang Z, Qin P, Deng Y et al (2018) The novel estrogenic receptor GPR30 alleviates ischemic injury by inhibiting TLR4-mediated microglial inflammation. J Neuroinflammation 15(1):206. https://doi.org/10.1186/s12974-018-1246-x
Jenny NS, Callas PW, Judd SE et al (2019) Inflammatory cytokines and ischemic stroke risk: the REGARDS cohort. Neurology 92(20):e2375–e2384. https://doi.org/10.1212/wnl.0000000000007416
Bis JC, Heckbert SR, Smith NL et al (2008) Variation in inflammation-related genes and risk of incident nonfatal myocardial infarction or ischemic stroke. Atherosclerosis 198(1):166–173. https://doi.org/10.1016/j.atherosclerosis.2007.09.031
Yuan S, Carter P, Bruzelius M et al (2020) Effects of tumour necrosis factor on cardiovascular disease and cancer: a two-sample Mendelian randomization study. EBioMedicine 59:102956. https://doi.org/10.1016/j.ebiom.2020.102956
Kang X, Jiao T, Wang H, Pernow J, Wirdefeldt K (2022) Mendelian randomization study on the causal effects of tumor necrosis factor inhibition on coronary artery disease and ischemic stroke among the general population. EBioMedicine 76:103824. https://doi.org/10.1016/j.ebiom.2022.103824
Kawada H, Takizawa S, Takanashi T et al (2006) Administration of hematopoietic cytokines in the subacute phase after cerebral infarction is effective for functional recovery facilitating proliferation of intrinsic neural stem/progenitor cells and transition of bone marrow-derived neuronal cells. Circulation 113(5):701–710. https://doi.org/10.1161/circulationaha.105.563668
Solaroglu I, Tsubokawa T, Cahill J, Zhang JH (2006) Anti-apoptotic effect of granulocyte-colony stimulating factor after focal cerebral ischemia in the rat. Neuroscience 143(4):965–974. https://doi.org/10.1016/j.neuroscience.2006.09.014
Guan ZF, Tao YH, Zhang XM et al (2017) G-CSF and cognitive dysfunction in elderly diabetic mice with cerebral small vessel disease: preventive intervention effects and underlying mechanisms. CNS Neurosci Ther 23(6):462–474. https://doi.org/10.1111/cns.12691
Calipari ES, Godino A, Peck EG et al (2018) Granulocyte-colony stimulating factor controls neural and behavioral plasticity in response to cocaine. Nat Commun 9(1):9. https://doi.org/10.1038/s41467-017-01881-x
Funding
This research was supported by grants 81772438, 81974357, and 82072548 from the National Science Foundation of China and grant 202206010197 from the Guangzhou Municipal Science and Technology Program
Author information
Authors and Affiliations
Contributions
G. B. C. and Y. L. designed and conceived the study. G. B. C., T. L., M. F. W., G. Y. C., Q. D., J. Y. X., C. W., and H. Y. C. contributed to data acquisition. G. B. C. and G. Q. X. supervised data entry and integrity. G. B. C. analyzed data and prepared the figures. G. B. C., Y. L., and G. Q. X. wrote the manuscript. All authors reviewed and approved the final draft.
Corresponding authors
Ethics declarations
Ethics Approval
All relevant ethics approvals are from original genome-wide association studies.
Consent to Participate
All data in the MR study were obtained from published GWAS studies, and the participants had signed informed consent in the published original GWAS study.
Consent for Publication
All data in the MR study were obtained from published GWAS studies, and the participants had signed informed consent in the published original GWAS study.
Conflict of Interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Chen, G., Lin, T., Wu, M. et al. Causal Association of Cytokines and Growth Factors with Stroke and Its Subtypes: a Mendelian Randomization Study. Mol Neurobiol 61, 3212–3222 (2024). https://doi.org/10.1007/s12035-023-03752-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12035-023-03752-7