Abstract
Perioperative neurocognitive disorders (PNDs) are severe and common neurological complications among elderly patients following anesthesia and surgery. As the first line of defense of the innate immune system, Toll-like receptors (TLRs) have been found to be involved in the occurrence of neurodegenerative diseases in recent years. However, the role of TLR7 in the pathology and development of PNDs remains largely unclear. In our current study, we hypothesized that increased microRNA let-7b (let-7b) during anesthesia and surgical operation would activate TLR7 signaling pathways and mediate PNDs. Using a mouse model of PNDs, 18–20 months wild-type (WT) mice were undergoing unilateral nephrectomy, and increased TLR7 and let-7b expression levels were found in the surgery group compared with the Sham group. Of note, increased TLR7 was found to be co-localized with let-7b in the hippocampal area CA1 in the PNDs model. In addition, TLR7 and let-7b inhibition could improve hippocampus-dependent memory and attenuate the production of inflammatory cytokines. Together, our results indicated that TLR7 activation and up-regulation might be triggered by increased let-7b under stressful conditions and initiated the downstream inflammatory signaling, playing a substantial role in the development of PNDs.







Similar content being viewed by others
Data Availability
The datasets used and/or analyzed during the present study are available from the corresponding author upon reasonable request.
References
Evered L, Silbert B, Knopman DS, Scott DA, DeKosky ST, Rasmussen LS, Oh ES, Crosby G, Berger M, Eckenhoff RG, Nomenclature Consensus Working G (2018) Recommendations for the nomenclature of cognitive change associated with anaesthesia and surgery-2018. Br J Anaesth 121(5):1005–1012. https://doi.org/10.1016/j.bja.2017.11.087
Evered L, Scott DA, Silbert B, Maruff P (2011) Postoperative cognitive dysfunction is independent of type of surgery and anesthetic. Anesth Analg 112(5):1179–1185. https://doi.org/10.1213/ANE.0b013e318215217e
Ballard C, Jones E, Gauge N, Aarsland D, Nilsen OB, Saxby BK, Lowery D, Corbett A, Wesnes K, Katsaiti E, Arden J, Amoako D, Prophet N, Purushothaman B, Green D (2012) Optimised anaesthesia to reduce post operative cognitive decline (POCD) in older patients undergoing elective surgery, a randomised controlled trial. PLoS ONE 7(6):e37410. https://doi.org/10.1371/journal.pone.0037410
Seitz DP, Shah PS, Herrmann N, Beyene J, Siddiqui N (2011) Exposure to general anesthesia and risk of Alzheimer’s disease: a systematic review and meta-analysis. BMC Geriatr 11:83. https://doi.org/10.1186/1471-2318-11-83
Barnes CA (1988) Aging and the physiology of spatial memory. Neurobiol Aging 9(5–6):563–568
Song S, Zhao W, Ji Y, Huang Q, Li Y, Chen S, Yang J, Jin X (2023) SHANK2 protein contributes to sevoflurane-induced developmental neurotoxicity and cognitive dysfunction in C57BL/6 male mice. Anesthesiol Perioper Sci 1(1):2. https://doi.org/10.1007/s44254-023-00005-7
Eichenbaum H (2001) The hippocampus and declarative memory: cognitive mechanisms and neural codes. Behav Brain Res 127(1–2):199–207
Miller DB, O’Callaghan JP (2005) Aging, stress and the hippocampus. Ageing Res Rev 4(2):123–140. https://doi.org/10.1016/j.arr.2005.03.002
Terrando N, Eriksson LI, Ryu JK, Yang T, Monaco C, Feldmann M, Jonsson Fagerlund M, Charo IF, Akassoglou K, Maze M (2011) Resolving postoperative neuroinflammation and cognitive decline. Ann Neurol 70(6):986–995. https://doi.org/10.1002/ana.22664
Balusu S, Van Wonterghem E, De Rycke R, Raemdonck K, Stremersch S, Gevaert K, Brkic M, Demeestere D, Vanhooren V, Hendrix A, Libert C, Vandenbroucke RE (2016) Identification of a novel mechanism of blood-brain communication during peripheral inflammation via choroid plexus-derived extracellular vesicles. EMBO Mol Med 8(10):1162–1183. https://doi.org/10.15252/emmm.201606271
Chen C, Gao R, Li M, Wang Q, Chen H, Zhang S, Mao X, Behensky A, Zhang Z, Gan L, Li T, Liao R, Li Q, Yu H, Yang J, Zhu T, Liu J (2019) Extracellular RNAs-TLR3 signaling contributes to cognitive decline in a mouse model of postoperative cognitive dysfunction. Brain Behav Immun 80:439–451. https://doi.org/10.1016/j.bbi.2019.04.024
Wang Y, He H, Li D, Zhu W, Duan K, Le Y, Liao Y, Ou Y (2013) The role of the TLR4 signaling pathway in cognitive deficits following surgery in aged rats. Mol Med Rep 7(4):1137–1142. https://doi.org/10.3892/mmr.2013.1322
Lu SM, Yu CJ, Liu YH, Dong HQ, Zhang X, Zhang SS, Hu LQ, Zhang F, Qian YN, Gui B (2015) S100A8 contributes to postoperative cognitive dysfunction in mice undergoing tibial fracture surgery by activating the TLR4/MyD88 pathway. Brain Behav Immun 44:221–234. https://doi.org/10.1016/j.bbi.2014.10.011
Gambuzza ME, Sofo V, Salmeri FM, Soraci L, Marino S, Bramanti P (2014) Toll-like receptors in Alzheimer’s disease: a therapeutic perspective. CNS Neurol Disord Drug Targets 13(9):1542–1558. https://doi.org/10.2174/1871527313666140806124850
Luo Z, Su R, Wang W, Liang Y, Zeng X, Shereen MA, Bashir N, Zhang Q, Zhao L, Wu K, Liu Y, Wu J (2019) EV71 infection induces neurodegeneration via activating TLR7 signaling and IL-6 production. PLoS Pathog 15(11):e1008142. https://doi.org/10.1371/journal.ppat.1008142
Areschoug T, Gordon S (2008) Pattern recognition receptors and their role in innate immunity: focus on microbial protein ligands. Contrib Microbiol 15:45–60. https://doi.org/10.1159/000135685
Fitzgerald KA, Kagan JC (2020) Toll-like receptors and the control of immunity. Cell 180(6):1044–1066. https://doi.org/10.1016/j.cell.2020.02.041
Krol J, Kaczynska D, Krzyzosiak WJ (2003) Micro RNA–members of non-coding RNA family. Postepy Biochem 49(4):214–228
Fleshner M, Crane CR (2017) Exosomes, DAMPs and miRNA: features of stress physiology and immune homeostasis. Trends Immunol 38(10):768–776. https://doi.org/10.1016/j.it.2017.08.002
Chen L, Dong R, Lu Y, Zhou Y, Li K, Zhang Z, Peng M (2019) MicroRNA-146a protects against cognitive decline induced by surgical trauma by suppressing hippocampal neuroinflammation in mice. Brain Behav Immun 78:188–201. https://doi.org/10.1016/j.bbi.2019.01.020
Lehmann SM, Kruger C, Park B, Derkow K, Rosenberger K, Baumgart J, Trimbuch T, Eom G, Hinz M, Kaul D, Habbel P, Kalin R, Franzoni E, Rybak A, Nguyen D, Veh R, Ninnemann O, Peters O, Nitsch R, Heppner FL, Golenbock D, Schott E, Ploegh HL, Wulczyn FG, Lehnardt S (2012) An unconventional role for miRNA: let-7 activates Toll-like receptor 7 and causes neurodegeneration. Nat Neurosci 15(6):827–835. https://doi.org/10.1038/nn.3113
Klammer MG, Dzaye O, Wallach T, Krüger C, Gaessler D, Buonfiglioli A, Derkow K, Kettenmann H, Brinkmann MM, Lehnardt S (2021) UNC93B1 is widely expressed in the murine CNS and is required for neuroinflammation and neuronal injury induced by MicroRNA let-7b. Front Immunol 12:715774. https://doi.org/10.3389/fimmu.2021.715774
Roush S, Slack FJ (2008) The let-7 family of microRNAs. Trends Cell Biol 18(10):505–516. https://doi.org/10.1016/j.tcb.2008.07.007
Lee H, Han S, Kwon CS, Lee D (2016) Biogenesis and regulation of the let-7 miRNAs and their functional implications. Protein Cell 7(2):100–113. https://doi.org/10.1007/s13238-015-0212-y
Coleman LG, Zou J, Crews FT (2017) Microglial-derived miRNA let-7 and HMGB1 contribute to ethanol-induced neurotoxicity via TLR7. J Neuroinflammation 14(1):22. https://doi.org/10.1186/s12974-017-0799-4
Sprung J, Roberts RO, Weingarten TN, Nunes Cavalcante A, Knopman DS, Petersen RC, Hanson AC, Schroeder DR, Warner DO (2017) Postoperative delirium in elderly patients is associated with subsequent cognitive impairment. Br J Anaesth 119(2):316–323. https://doi.org/10.1093/bja/aex130
Vizcaychipi MP, Watts HR, O’Dea KP, Lloyd DG, Penn JW, Wan Y, Pac-Soo C, Takata M, Ma D (2014) The therapeutic potential of atorvastatin in a mouse model of postoperative cognitive decline. Ann Surg 259(6):1235–1244. https://doi.org/10.1097/SLA.0000000000000257
Xiang Y, Bu X-L, Liu Y-H, Zhu C, Shen L-L, Jiao S-S, Zhu X-Y, Giunta B, Tan J, Song W-H, Zhou H-D, Zhou X-F, Wang Y-J (2015) Physiological amyloid-beta clearance in the periphery and its therapeutic potential for Alzheimer’s disease. Acta Neuropathol 130(4):487–499. https://doi.org/10.1007/s00401-015-1477-1
Balazsfi D, Fodor A, Torok B, Ferenczi S, Kovacs KJ, Haller J, Zelena D (2018) Enhanced innate fear and altered stress axis regulation in VGluT3 knockout mice. Stress 21(2):151–161. https://doi.org/10.1080/10253890.2017.1423053
Rao SS, Lago L, Volitakis I, Shukla JJ, McColl G, Finkelstein DI, Adlard PA (2021) Deferiprone treatment in aged transgenic tau mice improves Y-Maze performance and alters tau pathology. Neurotherapeutics 18(2):1081–1094. https://doi.org/10.1007/s13311-020-00972-w
Pugh CR, Kumagawa K, Fleshner M, Watkins LR, Maier SF, Rudy JW (1998) Selective effects of peripheral lipopolysaccharide administration on contextual and auditory-cue fear conditioning. Brain Behav Immun 12(3):212–229. https://doi.org/10.1006/brbi.1998.0524
Mukherjee S, Akbar I, Kumari B, Vrati S, Basu A, Banerjee A (2019) Japanese Encephalitis Virus-induced let-7a/b interacted with the NOTCH-TLR7 pathway in microglia and facilitated neuronal death via caspase activation. J Neurochem 149(4):518–534. https://doi.org/10.1111/jnc.14645
Lin F, Shan W, Zheng Y, Pan L, Zuo Z (2021) Toll-like receptor 2 activation and up-regulation by high mobility group box-1 contribute to post-operative neuroinflammation and cognitive dysfunction in mice. J Neurochem 158(2):328–341. https://doi.org/10.1111/jnc.15368
Dalpke A, Helm M (2012) RNA mediated Toll-like receptor stimulation in health and disease. RNA Biol 9(6):828–842. https://doi.org/10.4161/rna.20206
Petes C, Odoardi N, Gee K (2017) The toll for trafficking: toll-like receptor 7 delivery to the endosome. Front Immunol 8:1075. https://doi.org/10.3389/fimmu.2017.01075
Cowan M, Petri WA Jr (2018) Microglia: immune regulators of neurodevelopment. Front Immunol 9:2576. https://doi.org/10.3389/fimmu.2018.02576
Hickman S, Izzy S, Sen P, Morsett L, El Khoury J (2018) Microglia in neurodegeneration. Nat Neurosci 21(10):1359–1369. https://doi.org/10.1038/s41593-018-0242-x
Shastri A, Bonifati DM, Kishore U (2013) Innate immunity and neuroinflammation. Mediators Inflamm 2013:342931. https://doi.org/10.1155/2013/342931
Colonna M, Butovsky O (2017) Microglia function in the central nervous system during health and neurodegeneration. Annu Rev Immunol 35:441–468. https://doi.org/10.1146/annurev-immunol-051116-052358
Heppner FL, Greter M, Marino D, Falsig J, Raivich G, Hovelmeyer N, Waisman A, Rulicke T, Prinz M, Priller J, Becher B, Aguzzi A (2005) Experimental autoimmune encephalomyelitis repressed by microglial paralysis. Nat Med 11(2):146–152. https://doi.org/10.1038/nm1177
Lucas SM, Rothwell NJ, Gibson RM (2006) The role of inflammation in CNS injury and disease. Br J Pharmacol 147(Suppl 1):S232-240. https://doi.org/10.1038/sj.bjp.0706400
Liu HY, Hong YF, Huang CM, Chen CY, Huang TN, Hsueh YP (2013) TLR7 negatively regulates dendrite outgrowth through the Myd88-c-Fos-IL-6 pathway. J Neurosci 33(28):11479–11493. https://doi.org/10.1523/JNEUROSCI.5566-12.2013
Letiembre M, Hao W, Liu Y, Walter S, Mihaljevic I, Rivest S, Hartmann T, Fassbender K (2007) Innate immune receptor expression in normal brain aging. Neuroscience 146(1):248–254. https://doi.org/10.1016/j.neuroscience.2007.01.004
Mukherjee S, Huda S, Sinha Babu SP (2019) Toll-like receptor polymorphism in host immune response to infectious diseases: a review. Scand J Immunol 90(1):e12771. https://doi.org/10.1111/sji.12771
Heil F, Hemmi H, Hochrein H, Ampenberger F, Kirschning C, Akira S, Lipford G, Wagner H, Bauer S (2004) Species-specific recognition of single-stranded RNA via toll-like receptor 7 and 8. Science 303(5663):1526–1529. https://doi.org/10.1126/science.1093620
Diebold SS, Kaisho T, Hemmi H, Akira S, Reis e Sousa C (2004) Innate antiviral responses by means of TLR7-mediated recognition of single-stranded RNA. Science 303(5663):1529–1531. https://doi.org/10.1126/science.1093616
Achek A, Yesudhas D, Choi S (2016) Toll-like receptors: promising therapeutic targets for inflammatory diseases. Arch Pharm Res 39(8):1032–1049. https://doi.org/10.1007/s12272-016-0806-9
Ohashi K, Burkart V, Flohe S, Kolb H (2000) Cutting edge: heat shock protein 60 is a putative endogenous ligand of the toll-like receptor-4 complex. J Immunol 164(2):558–561. https://doi.org/10.4049/jimmunol.164.2.558
Dinger ME, Mercer TR, Mattick JS (2008) RNAs as extracellular signaling molecules. J Mol Endocrinol 40(4):151–159. https://doi.org/10.1677/JME-07-0160
Kluever AK, Deindl E (2018) Extracellular RNA, a potential drug target for alleviating atherosclerosis, ischemia/reperfusion injury and organ transplantation. Curr Pharm Biotechnol 19(15):1189–1195. https://doi.org/10.2174/1389201020666190102150610
Feng Y, Zou L, Yan D, Chen H, Xu G, Jian W, Cui P, Chao W (2017) Extracellular microRNAs induce potent innate immune responses via TLR7/MyD88-dependent mechanisms. J Immunol 199(6):2106–2117. https://doi.org/10.4049/jimmunol.1700730
Chen C, Cai J, Zhang S, Gan L, Dong Y, Zhu T, Ma G, Li T, Zhang X, Li Q, Cheng X, Wu C, Yang J, Zuo Y, Liu J (2015) Protective effect of RNase on unilateral nephrectomy-induced postoperative cognitive dysfunction in aged mice. PLoS ONE 10(7):e0134307. https://doi.org/10.1371/journal.pone.0134307
Derkow K, Rossling R, Schipke C, Kruger C, Bauer J, Fahling M, Stroux A, Schott E, Ruprecht K, Peters O, Lehnardt S (2018) Distinct expression of the neurotoxic microRNA family let-7 in the cerebrospinal fluid of patients with Alzheimer’s disease. PLoS ONE 13(7):e0200602. https://doi.org/10.1371/journal.pone.0200602
Moulton JD, Jiang S (2009) Gene knockdowns in adult animals: PPMOs and vivo-morpholinos. Molecules 14(3):1304–1323. https://doi.org/10.3390/molecules14031304
Nazmi A, Mukherjee S, Kundu K, Dutta K, Mahadevan A, Shankar SK, Basu A (2014) TLR7 is a key regulator of innate immunity against Japanese encephalitis virus infection. Neurobiol Dis 69:235–247. https://doi.org/10.1016/j.nbd.2014.05.036
O’Neill LA, Bowie AG (2007) The family of five: TIR-domain-containing adaptors in Toll-like receptor signalling. Nat Rev Immunol 7(5):353–364. https://doi.org/10.1038/nri2079
Laffont S, Seillet C, Guery JC (2017) Estrogen receptor-dependent regulation of dendritic cell development and function. Front Immunol 8:108. https://doi.org/10.3389/fimmu.2017.00108
Hung YF, Chen CY, Li WC, Wang TF, Hsueh YP (2018) Tlr7 deletion alters expression profiles of genes related to neural function and regulates mouse behaviors and contextual memory. Brain Behav Immun 72:101–113. https://doi.org/10.1016/j.bbi.2018.06.006
Acknowledgements
The authors would like to thank and express their heartfelt gratitude to Hongying Chen, Yan Wang, Huifang Li, Xiangyi Ren, Mengli Zhu, Li Fu, and Cong Li. (Cytology and Molecular Platform, Core Facilities of West China Hospital) for their assistance and guidance.
Funding
This work was supported by the National Natural Science Foundation of China (No.82171185, No. 81870858 to Dr. Chan Chen); The National Key R&D Program of China (No. 2018YFC2001800, to Dr. Tao Zhu) and the National Natural Science Foundation of China (No. 81671062, to Dr. Tao Zhu); The National Natural Science Foundation of China (No. 82201426 to Rui Gao); The Natural Science Foundation of Sichuan Province (No. 2022NSFSC1322 to Rui Gao); The China Postdoctoral Science Foundation (No. 2020 M673234 to Rui Gao); The Postdoctoral Research Project, West China Hospital, Sichuan University (No. 2020HXBH022 to Rui Gao).
Author information
Authors and Affiliations
Contributions
Conceptualization: Liyun Deng, Rui Gao, and Chan Chen; Methodology: Bo Jiao and Changteng Zhang; Formal analysis and investigation: Liyun Deng, Liuxing Wei, and Caiyi Yan; Writing—original draft preparation: Liyun Deng, Rui Gao; Writing—review and editing: Chan Chen and Tao Zhu; Funding acquisition: Rui Gao, Tao Zhu, and Chan Chen; Resources: Hai Chen, Shixin Ye-Lehmann; Supervision: Tao Zhu, Chan Chen. All authors reviewed the manuscript.
Corresponding author
Ethics declarations
Ethics Approval
This study was performed in line with the principles of the Declaration of Helsinki. Approval was granted by the Animal Care and Use Committee of Sichuan University (Permit Number: 2021625A).
Consent to Participate
Not applicable.
Consent for Publication
Not applicable.
Competing Interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.

Supplement Figure 1
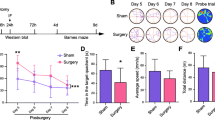
Changes in cognitive function at different days after unilateral nephrectomy. a Percentage of freezing time in the fear conditioning test of aged mice on the 1st, 3rd, and 7th day after unilateral nephrectomy. b Percentage of novel arm time in the Y maze test of aged mice on the 1st, 3rd, and 7th day after unilateral nephrectomy. c Mean velocity in open field test of aged mice on the 1st, 3rd, and 7th day after unilateral nephrectomy. d The number of rearing in open field test of aged mice on the 1st, 3rd, and 7th day after unilateral nephrectomy. e Total travel distance in open field test of aged mice on the 1st, 3rd, and 7th day after unilateral nephrectomy. Data are presented as mean ± SEM (n = 6 per group).(PNG 81 kb)

Supplement Figure 2
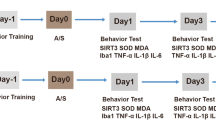
The stereotactic injection and decreased expression of TLR7 did not influence the cognitive function of aged mice in the physiological state. a Schematic outline of the experimental protocol. b. The representative image of the needle track and distribution of the mouse receiving injection of 2μl Evans blue in the CA1 of each hemisphere. On the right side, the injection channel can be identified. c Percentage of freezing time and representative chart fear conditioning test experiment. d Percentage of novel arm time in Y-maze. e Mean velocity, number of rearing, and total travel distance in open field test of aged mice following unilateral nephrectomy. Data are presented as mean ± SEM (n = 6 per group).(PNG 289 kb)
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Deng, L., Gao, R., Chen, H. et al. Let-7b-TLR7 Signaling Axis Contributes to the Anesthesia/Surgery-Induced Cognitive Impairment. Mol Neurobiol 61, 1818–1832 (2024). https://doi.org/10.1007/s12035-023-03658-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12035-023-03658-4