Abstract
Purpose
Repeated exposures to sevoflurane could induce epigenetic modifications in specific brain regions and cognitive impairments in the immature mice. Conflicting findings make neurobehavioral manifestations intricate and potential mechanisms elusive. Influence of neonatal anesthesia with sevoflurane on the expression of synaptic scaffold proteins and neuronal activity remains to be determined.
Methods
C57BL/6 male and female mice in breeding ages were used to produce next generation. The offspring male mice were randomly scheduled to receive 3.0% sevoflurane plus 60% oxygen for 2 h daily at postnatal day (P) 6–8. Three-chambered social paradigm was used to test social affiliation and social memory. Morris water maze was used to test learning and memory. Whole genome bisulfite sequencing (WGBS), differentially methylated regions (DMRs) and KEGG enrichment analysis were performed to screen target gene in sequence context of CG. RT-PCR and immunoblotting analysis were used to assess expression of the Shank gene family, as well as DNA methylases.
Results
The male mice undergoing sevoflurane anesthesia at P6-8 showed diminished preference for novel conspecific and prolonged escape latency and decreased platform-crossing times. The sevoflurane-exposed mice showed reduced mRNA and protein levels of the Shank2 gene. KEGG analysis disclosed the role of DNA hypermethylation of Shank2 gene in the pathway of glutamatergic synapse. In addition, sevoflurane anesthesia reduced mRNA and protein levels of the TET3 enzyme.
Conclusion
Repeated exposures to sevoflurane in neonatal period could impair social recognition memory and spatial reference memory in the male mice. Reduction of hippocampal SHANK2 protein could contribute to sevoflurane-induced neurotoxicity in the immature mice. Reduction of the TET3 enzyme should be responsible for DNA hypermethylation-related silencing of the Shank2 gene.
Graphical Abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Anesthesia facilitates diverse surgical procedures by alleviating intensity of pain. However, the patients are immersed in some pathophysiological processes, and the hazard depends on anesthetic regimen and patient fragility. Children aged less than 36 months might be more vulnerable to anesthesia for longer duration (≥ 3 h) and for multiple times (≥ 3 times) [1], with high risks of long-term neurobehavioral abnormalities [2]. In recent decades, anesthetic-induced developmental neurotoxicity has attracted much attention of physicians [3]. Nonetheless, the neuropathological mechanism remains uncertain.
Sevoflurane is supposed to induce neurotoxicity in immature brain and cause long-term cognitive dysfunction [4]. The mechanisms included neuroinflamamtion [5], neuroapoptosis [6, 7], synaptic ultrastructure alternation [8] and mitochondrial energy disturbance [9] in central nervous system. It was reported that epigenetic alterations including DNA methylation, histone modifications, and RNA interference, could influence diverse genes, causing neuronal and behavioral changes in mental disorders [10]. Precise regulation of DNA methylation in developing brain is essential for normal cognitive function [11]. Risk factors including drug exposure and neural injury could also change the process of DNA methylation and cause mental impairments. These studies suggested an epigenetic mechanism of cognitive dysfunction after sevoflurane anesthesia. It remains unclear what kind of proteins and epigenetic alterations could contribute to sevoflurane-induced cognitive dysfunction.
Sevoflurane-induced neurotoxic effects are associated with the family of gamma-aminobutyric acid (GABA) and N-Methyl-D-aspartate (NMDA) receptors. However, the onset and development of cognitive dysfunction could not be well explained by the approach of one-protein and one-disease. Clinical phenotypes of neurodevelopmental disorders might result from regulative interplay of diverse genes and proteins. The alterations of SHANK proteins, encoded by Shank1, Shank2, and Shank3, are associated with multiple neurodevelopmental disorders including autism spectrum disorders (ASD) and intellectual disability [12]. Previous studies demonstrated reduced PSD95 levels in the hippocampus of neonatal mice after sevoflurane anesthesia [9, 13, 14]. NMDA-type glutamate receptors in hippocampal synapses could bind to SHANK proteins in the PDZ domain through the PSD95/GKAP/SAP90-associated proteins (SAPAPs) [12], suggesting the SHANK proteins could serve as the synaptic scaffold proteins for PSD95-NMDAR complex. However, it remains to be determined whether sevoflurane anesthesia could change mRNA and protein levels of the Shank genes.
Therefore, we hypothesized that sevoflurane could down-regulate SHANK proteins which is essential to glutamatergic receptors, neuronal activity and neurobehavioral performances. To verify this hypothesis, we performed sevoflurane anesthesia for three times in neonatal mice, and test social memory in the three-chambered social paradigm and working memory in the Morris water maze. To determine the underlying mechanism, we measured levels of the SHANK proteins, analyzed epigenetic alternations of the Shank genes, and explored DNA methylases.
2 Methods
2.1 Animals
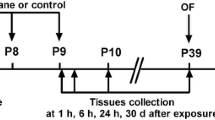
This study was approved by the Institutional Animal Care and Use Committee at Soochow University (Suzhou, Jiangsu, China). Research fellows made best effect to minimize the number of animals. Twenty-five female and five male C57BL/6 mice in breeding ages were purchased from Zhaoyan Laboratory (Taicang, Jiangsu, China), these mice were arranged to produce next generation. The offspring mice were treated with sevoflurane anesthesia at postnatal day 6, 7 and 8 (P6-8), and then housed 4–5 mice per cage after weaning at P21. Behavioral tests and harvest of the brain tissues were arranged at P30-37 (Fig. 1A). Littermates were randomized into two groups, and only male mice were used in this study. All mice were raised with free access to food and water in a standard environment: room temperature 21–22℃, 12/12 h light/dark cycle (light on at 7 am).
Neonatal exposure to sevoflurane for three times disturbs social recognition memory in the three-chambered social test and impairs learning and memory in the MWM in the juvenile male mice. A. Experimental design. Sevoflurane anesthesia is conducted in the neonatal mice at postnatal day (P) 6–8. Behavioral tests are performed in the juvenile mice at P30-36. Brain tissues of mouse hippocampus are harvested in the mice at P37. In the sociability test, the subject mice undergoing either sevoflurane anesthesia or control condition show strong social affiliation, as proved by spending more time sniffing B. and sniffing for more times C. at the enclosure with Stranger 1, as compared with the opposite empty enclosure. In the social novelty test, the subject mice undergoing sevoflurane anesthesia in neonatal period show weak social recognition, as evidenced by not preferring the Stranger 2 to Stranger 1 mouse in either sniffing time D. and sniffing times E. F. The subject mice undergoing sevoflurane anesthesia show increased escape latency tested in MWM, as compared with the control condition. G. The subject mice undergoing sevoflurane anesthesia show decreased platform-crossing times, as compared with the control condition. H. The subject mice undergoing sevoflurane anesthesia show less time swimming in the fourth quadrant, as compared with the control condition. I. The subject mice show no significant difference between two groups in the swimming speed. Data are expressed as Mean ± SD or Median [IQR]. N = 10 mice per group. Paired t-test B-E. Two-way RM ANOVA with Bonferroni’s multiple comparisons test F. Mann–Whitney U-test G. Student’s t-test H. I. *P < 0.05, **P < 0.01, ***P < 0.001. MWM, Morris water maze. SD, standard deviation. IQR, interquartile range. RM, repeated measures. NS, not significant
2.2 Anesthesia
Sevoflurane anesthesia was carried out with a retired anesthesia machine and the concentrations of sevoflurane and oxygen were adjusted by a gas analyzer (Datex-Ohmeda, Inc.). A sealed plastic box (20 L × 20 W × 6 H cm) was used as the anesthetizing chamber, with three holes for gas inflow, gas outflow and sampling respectively. An electric heating pad was placed underneath the anesthetizing chamber to keep mice warm during anesthesia. The neonatal mice were randomized to receive 3.0% sevoflurane plus 60% oxygen for 2 h daily at P6-8 (Sevo × 3) or merely 60% oxygen for 2 h daily at P6-8 (Control). In this study, 60% oxygen was adequate to guarantee oxygenation of neonatal mice during sevoflurane anesthesia with spontaneous respiration, with blood-gas analysis within normal limits as described in previous study [9]. Finally, sevoflurane was flushed with 60% oxygen for 15 min after anesthesia, and these mice were sent back to their own dams for maternal fostering.
2.3 Social interaction test
Three-chambered social paradigm was used to test social behaviors of the mice at P30, which consisted of three 10-min sessions, Habituation, Sociability (social affiliation) and Preference for social novelty (social memory) [13, 15]. Sociability is defined as the subject mouse prefers to sniff the conspecific (Stranger 1) rather than to contact an inanimate object (Enclosure). Preference for social novelty is defined as the subject mouse prefers to sniff the newly-introduced conspecific (Stranger 2) rather than to contact the already-familiar mouse (Stranger 1). A video-tracking system programmed by ANY-maze (Stoelting Co., USA) was used to capture mouse movement. In the first session, the testing mouse was allowed to freely explore in three chambers to get familiar with two enclosures. In the second session, the testing mouse was allowed to freely explore in three chambers and actively sniff the enclosure containing a conspecific mouse (Stranger 1) or the opposite empty enclosure. In the third session, the testing mouse was allowed to freely explore in three chambers and actively sniff the enclosure containing Stranger 1 or the opposite enclosure containing a newly-introduced mouse (Stranger 2). Stranger 1 was placed on the left or right side in turns. The stranger mice were the same strain, age and sex as the testing mice, with exactly no prior contact. The amount of time sniffing and number of sniffs at the enclosure were calculated to determine side preference.
2.4 Morris water maze (MWM) test
The male mice undergoing sevoflurane anesthesia at P6-8 were tested in the MWM at P31-36, with the protocols described in previous study [16]. The subject mice were firstly scheduled to reference training four times per day to search for the platform at P31-35. Next, the platform was removed from pool and the water was opacified with titanium dioxide, these mice were arranged to freely swim for 90 s at P36. The parameters including escape latency, platform-crossing times, time in the fourth quadrant and mean swimming speed were calculated to determine learning and memory.
2.5 Hippocampus preparation
To explore molecular mechanisms underlying neurobehavioral changes of the sevoflurane-treated mice, the mouse hippocampus was harvested at P37, with time point in close to behavioral testing. First of all, the mice were treated with 3.0% sevoflurane for 5 min (i.e. animal welfare). After losing consciousness, these mice were rapidly decapitated. Next, the brain tissues of hippocampus were harvested on the dry ice and stored in the -80℃ refrigerator. A tiny piece of hippocampus was homogenized in the lysis buffer, consisting of immunoprecipitation buffer plus protease inhibitor cocktail (Biocolors bioscience). And then, the upper lysates were collected and centrifuged at 1500 rpm for 15 min. Finally, the amounts of total protein were quantified by a BCA Protein Assay Kit (Beyotime Biotechnology).
2.6 Reverse Transcriptase-Polymerase Chain Reaction (RT-PCR)
The effects of sevoflurane anesthesia on mRNA levels of the Shank gene family and DNA methylases were determined by RT-PCR. RNA was harvested and isolated from the P37 mouse hippocampus, and RNA concentrations were determined by the NanoDrop-2000 Spectrophotometer (Thermo Scientific, USA). Next, the RNA was reversed to cDNA by using the 5X All-In-One RT MasterMix (Cat.#G490, abm). And then, cDNA was amplified by using the PerfectStart™ Green qPCR SuperMix (Lot#20,609, TRANS). After that, the attached fluorescent DyeII was used to detect amount of the cDNA, which was expressed as cycle time (CT, the time to detect dye-carried fluorescence bound to the cDNA). Finally, the CT values were converted into the amount of mRNA using a standard curve, with the glyceraldehyde 3-phosphate dehydrogenase (GAPDH) as an internal baseline. Primers of the Shank gene family and DNA methylases (Supplemental Table S1) were purchased from Sangon Biothec (Shanghai, China).
2.7 Western blot analysis
Western-blot analysis of protein levels and quantification of immunoblotting were performed by the protocols. Anti-SHANK2 antibody (catalog no. ab259966, 1:1,000, Abcam) was used to recognize SHANK2 (165 kDa). TET1 antibody (catalog no. sc-163443, 1:100, Santa) was used to recognize TET1 (235 kDa). TET2 antibody (catalog no. ab124297, 1:1,000, Abcam) was used to recognize TET2 (223 kDa). TET3 antibody (catalog no. sc-139186, 1:100, Santa) was used to recognize TET3 (179 kDa). Anti-GAPDH antibody (catalog no. 85–14-9523–82, 37 kDa, 1:1,000, Multisciences, Biotech) was used to recognize non-targeted protein GAPDH (37 kDa). Quantification of Western blots was performed as follows. Firstly, GAPDH levels were used to normalize the targeted protein levels by determining the amount ratio of targeted protein to GAPDH, which was regarded as the control for loading differences of total protein amount. Next, the targeted protein levels were presented as a percentage of those in the control. One hundred percent of protein level referred to the control level, serving for comparison with sevoflurane anesthesia at P6-8.
2.8 Electrophysiological recordings
Preparation of mouse brain slices and whole cell patch-clamp recordings of hippocampal CA1 neurons were performed by following protocols [17,18,19]. Eight P37 male mice undergoing sevoflurane anesthesia or control condition (N = 4 per group) were anesthetized with 3.0% sevoflurane for 5 min. Next, transverse brain slices of mouse hippocampus (400 μm) were prepared by standard methods with oxygenated (95% O2, 5% CO2) solution (mM): N-Methyl-D-glucamine (NMDG), 93; KCl, 2.5; NaH2PO4, 1.2; NaHCO3, 30; 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid (HEPES), 20; sodium ascorbate, 5; thiourea, 2; sodium pyruvate, 3; NAC, 12; MgSO4, 10; CaCl2, 0.5; and glucose, 25; at temperature 32 °C. Ten minutes later, the brain slices were transferred into oxygenated holding solution (mM): NaCl, 94; KCl, 2.5; NaH2PO4, 1.2; NaHCO3, 30; HEPES, 20; sodium ascorbate, 5; thiourea, 2; sodium pyruvate, 3; MgSO4, 2; CaCl2, 2; NAC, 12; and glucose, 25; at room temperature. One hour later, these brain slices were transported to recording chamber and perfused with ACSF (mM): NaCl, 124; KCl, 2.5; NaH2PO4, 1.2; NaHCO3, 24; HEPES, 5; glucose, 12.5; MgSO4, 2; and CaCl2, 2. Whole cell patch-clamp recordings of hippocampal CA1 pyramidal neurons were visualized by using infrared differential interference contrast (IR-DIC) video microscopy with a 40 × magnification water-immersion objective (BX51WI, Olympus, Japan). Spontaneous excitatory postsynaptic currents (sEPSCs) were recorded by a Digidata 1440A interface and MultiClamp 700B (Axon Instruments, CA, USA) amplifier in voltage-clamp mode. Membrane potential was kept at -70 mV for EPSC recording. Electrode (4–8 MΩ tip resistance) internal solution contained (mM): K-gluconate, 133; NaCl, 8; EGTA, 0.6; HEPES, 10; Mg-ATP, 2; and Na-GTP, 0.3. Series resistance (< 20 MΩ) was frequently checked to guarantee high-quality recordings. Signals were sampled and filtered at 10 kHz. Data were stored on the computer using pCLAMP 10 software (Axon Instruments, CA, USA) and analyzed with Mini Analysis (Synaptosoft, GA, USA).
2.9 Whole Genome Bisulfite Sequencing (WGBS)
Six P37 male mice undergoing sevoflurane anesthesia or control condition (N = 3 per group) were used for whole genome bisulfite sequencing (WGBS). Firstly, the DNAs were extracted from hippocampal tissues and tested for sample quality in the concentration and integrity. Next, genomic DNAs were fragmented into 100–300 bp by Sonication (Covaris, MA, USA) and purified with MiniElute PCR Purification Kit (QIAGEN, MD, USA). And then, the fragmented DNAs were repaired by adding a single “A” nucleotide to the 3’ end of blunt fragments, and the genomic fragments were ligated to methylated sequencing adapters. After that, bisulfite treatment was performed with Methylation-Gold kit (ZYMO, CA, USA), by which the unmethylated cytosine was converted to uracil. Finally, the converted DNA fragments were PCR-amplified and sequenced using Illumina HiSeqTM 2500 by Gene Denovo Biotechnology Co. (Guangzhou, China).
The methylation level was calculated based on the percentage of methylated cytosine to the whole genome, in different regions of the genome for each sequence context of CG, CHG and CHH (H representing A/C/T). To assess different methylation patterns in different genomic regions, the methylation profile at flanking 2 kb regions and gene body was plotted based on the average methylation levels for each window. To identify differentially methylated regions (DMRs) between hippocampal samples, the minimum read coverage to call a methylation status for a base was set to 4. The criteria of DMRs for sequence context of CG were as follows: numbers of CG in each window ≥ 5, absolute value of the difference in methylation ratio ≥ 0.25, and q ≤ 0.05. Kyoto Encyclopedia of Genes and Genomes (KEGG) pathway enrichment analysis was used to explore physiological function of the differentially methylated genes.
2.10 Statistical analysis
Data were expressed as mean ± SD or Median (IQR). Graphpad Prism 9.0 (San Diego, USA) was used for Statistical analysis and plotting. Sample sizes of the mice for behavioral testing were 10 in each group. Sample sizes of the hippocampi for RT-PCR and Western-blot were 4–5 in each group. Distributions of the data were checked by Kolmogorov–Smirnov tests. Paired t-test was used to analyze side preference in social interaction test. Two-way repeated measures (RM) ANOVA with Bonferroni's multiple comparisons was used to analyze escape latency, and Mann–Whitney U-test was used to analyze platform-crossing times in MWM test. Student’s t-test was used to determine differences of time in the fourth quadrant and swimming speed in MWM, mRNA levels in RT-PCR, and protein levels in Western-blot. Pearson's chi-square test (χ2) in methyl-Kit (version 1.7.10) was used to determine differential DNA methylation between samples at each locus. P values less than 0.05 (*), 0.01 (**) and 0.001 (***) were considered statistically significant.
3 Results
3.1 Sevoflurane anesthesia at P6-8 disturbed social recognition memory of the P30 mice tested in the three-chambered social paradigm and impaired learning and memory of the P31-36 mice tested in the MWM
In the three-chambered social test, there were not significant differences between the left and right side during 10-min habituation, in the amount of time sniffing and the number of sniffs at two empty enclosures (data not shown). The subject mice undergoing sevoflurane anesthesia in neonatal period showed strong sociability, as proved by spending more time sniffing the Stranger 1 mouse (Fig. 1B, Stranger 1 vs. Empty side; 152.2 ± 74.0 vs. 60.2 ± 20.5 s, P = 0.01 Control; 167.1 ± 45.6 vs. 65.2 ± 39.4 s, P < 0.0001 Sevo × 3; paired t-test), and approaching more frequently to the enclosure containing Stranger 1 (Fig. 1C; 62.6 ± 30.8 vs. 30.5 ± 9.2, P = 0.0201 Control; 68.2 ± 13.2 vs. 31.9 ± 11.8, P < 0.0001 Sevo × 3). Based on the significant side preference in sociability test, these subject mice had become sufficiently familiar with Stranger 1. However, these mice showed weak preference for social novelty, as evidenced by not spending more time sniffing the Stranger 2 mouse (Fig. 1D, Stranger 1 vs. Stranger 2; 65.0 ± 24.3 vs. 149.9 ± 56.1 s, P = 0.0006 Control; 95.5 ± 34.9 vs. 108.5 ± 27.3 s, P = 0.3588 Sevo × 3), and not approaching frequently to the enclosure containing Stranger 2 (Fig. 1E; 33.9 ± 11.5 vs. 55.1 ± 22.2, P = 0.0074 Control; 45.8 ± 16.7 vs. 47.6 ± 10.9, P = 0.6575 Sevo × 3).
In the MWM test, there was a significant main effect of treatment (Control vs. Sevo × 3) in the amount of escape latency (Fig. 1F; F (1, 18) = 26.61, P < 0.0001), and the multiple comparisons test showed increased amounts of escape latency at P33-35. Moreover, the P36 mice undergoing sevoflurane anesthesia showed less times of crossing platform (Fig. 1G, Control vs. Sevo × 3; 5.5 [4.0, 7.3] vs. 3.0 [1.8, 5.3], P = 0.0126) and less time swimming in the fourth quadrant (Fig. 1H; 11.9 ± 3.3 vs. 7.05 ± 4.36 s P = 0.0113). There was no significant difference between two groups in the swimming speed (Fig. 1I; P = 0.5544). Taken together, these findings suggested that neonatal anesthesia with sevoflurane for three times could disturb social recognition memory and impair learning and memory in the juvenile mice.
3.2 Whole transcriptome RNA-sequencing disclosed down-regulation of the gene in hippocampus of the mice undergoing sevoflurane anesthesia at P6-8
Considering importance of the hippocampus in learning, memory and cognition [20], and neurobehavioral changes in sevoflurane-treated mice, we performed whole transcriptome RNA sequencing (RNA-seq) in hippocampus of the P37 mice. A total number of 314 differentially expressed genes (DEGs) were detected in hippocampus of the mice undergoing sevoflurane anesthesia, including 49 up-regulated genes and 265 down-regulated genes as described in our previous study [21]. Furthermore, gene ontology (GO) analysis indicated down-regulation of the Shank2 gene, which was specifically associated with learning, memory, and social behavior (Table 1).
3.3 Sevoflurane anesthesia at P6-8 decreased transcription and translation of the Shank2 gene and reduced amplitude and frequency of neuronal sEPSCs in hippocampus of the P37 mice
Given that the Shank2 gene was down-regulated in hippocampus of the P37 mice treated with sevoflurane anesthesia at P6-8, we checked mRNA levels of the Shank family in RT-PCR, and found that sevoflurane was able to decrease mRNA levels of the Shank2 gene (Fig. 2B, Control vs. Sevo × 3; 100.0% ± 33.5% vs. 46.2% ± 27.3%, P = 0.0237, Student’s t-test). Meanwhile, there were not significant changes of mRNA levels of the Shank1 (Fig. 2A; 100.0% ± 54.7% vs. 82.6% ± 16.9%, P = 0.5152) and Shank3 (Fig. 2C; 100.0% ± 29.4% vs. 112.8% ± 21.3%, P = 0.452) genes. Next, we performed Western-blot to determine the effect of sevoflurane anesthesia on the SHANK2 protein. Immunoblotting analysis showed sevoflurane anesthesia at P6-8 decreased protein levels of SHANK2 (Fig. 3D and E; 100.0% ± 10.0% vs. 81.1% ± 11.8%, P = 0.0256) in comparison to the control condition. In addition, we performed whole cell patch-clamp recordings and found sevoflurane anesthesia reduced the amplitude (Fig. 2F, G and I; -8.7 ± 0.8 vs. -6.1 ± 1.1 pA, P = 0.0001) and frequency (Fig. 2F, H and J; 5.3 ± 1.5 vs. 3.9 ± 1.1 Hz, P = 0.0459) of sEPSCs in the hippocampal CA1 pyramidal neurons. Collectively, these findings suggested that sevoflurane anesthesia in neonatal period could decrease transcription and translation of the Shank2 gene, and reduce hippocampal neuronal activity in the mice.
Neonatal exposure to sevoflurane for three times (Sevo×3) decreases transcription and translation of the Shank2 gene in mouse hippocampus and reduces spontaneous excitatory postsynaptic currents (sEPSCs) in the hippocampal CA1 pyramidal neurons. RT-PCR analysis shows that sevoflurane anesthesia decreases mRNA levels of Shank2 B. but not Shank1 A. or Shank3 C. in mouse hippocampus. D. Western-blot analysis shows that sevoflurane anesthesia decreases the protein levels of SHANK2. There is no statistically significant difference in the amounts of GAPDH in hippocampus of the mice undergoing sevoflurane anesthesia or control condition. E. Quantification of immunoblotting shows that sevoflurane anesthesia decreases hippocampal SHANK2 levels, as compared with the control condition (N = 5 per group). F. Representative traces of sEPSCs recorded in the hippocampal CA1 pyramidal neurons of the mice undergoing sevoflurane anesthesia or control condition. Quantification of whole-cell current traces shows that sevoflurane anesthesia reduces the amplitude G. and frequency I. of sEPSCs in hippocampal neurons, as compared with the control condition (N = 8 cells per group). Cumulative probability distributions of sEPSCs amplitude H. and frequency J. are presented. Data were expressed as Mean ± SD. Student’s t-test. *P < 0.05; ***P < 0.001. sEPSCs, spontaneous excitatory postsynaptic currents
Differentially methylated regions (DMRs) analysis in the whole genome bisulfite sequencing (WGBS). A. Kernel density plotting of CG methylation. Two curves representing the efficiency of bisulfite conversion of nucleotides from the unmethylated cytosine to uracil. B. Statistic analysis of differentially methylated regions showing 150 up-regulated and 450 down-regulated DMRs for the sequence context of CG. C. Heatmap visualization of head 100 differentially methylated regions. Color is labeled as the intensity of differential methylation from lowest (blue) to highest (red). D. KEGG enrichment analysis showing top 20 signaling pathways. Color representing q-value from lowest (red) to highest (blue) and bubble size representing the significantly shifted gene number in individual pathway
3.4 Whole genome bisulfite sequencing in hippocampus of the sevoflurane-treated mice disclosed differentially methylated regions for sequence context of CG in the Shank2 gene which was involved in the pathway of glutamatergic synapse
Since sevoflurane anesthesia could decrease mRNA and protein levels of the Shank2 gene, we asked the underlying mechanism of Shank2 down-regulation. Thereby, we performed WGBS and DMRs analysis for sequence context (CG, CHG and CHH) to determine epigenetic alternation. The efficiency of bisulfite treatment was essential for determining positions of DNA methylation, and the converting ratios of six samples (N = 3 in each group) were 0.98974, 0.989946 and 0.973777 (Sevo × 3), 0.987824, 0.985769, and 0.985809 (control), which indicated a reliable bisulfite conversion of the unmethylated cytosine to uracil (Fig. 3A). Statistical analysis indicated 600 differentially methylated regions in the genomic DNAs for sequence context of CG, including 150 up-regulated and 450 down-regulated DMRs (Fig. 3B). GO analysis disclosed differentially methylated regions in the Shank2 gene (GeneID: ENSMUSG00000037541) (Fig. 3C and supplemental Table S2). KEGG pathway enrichment analysis disclosed top 20 pathways, with the shank2 gene listed in the pathway of glutamatergic synapse (Fig. 3D and supplemental Table S3).
3.5 Sevoflurane anesthesia at P6-8 decreased mRNA levels of the Tet genes and reduced protein levels of the TET3 enzyme in hippocampus of the P37 mice
As the Shank2 gene was detected of differentially methylated regions for sequence context of CG, we performed RT-PCR to explore up-stream mechanism of DNA methylation. There were not significant changes between two groups in mRNAs levels of the DNA methylases, including DNA methyltransferases-1 (DNMT1) (Fig. 4A; P = 0.6938), DNMT3a (Fig. 4B; P = 0.5189) or DNMT3b (Fig. 4C; P = 0.2991) which could promote DNA methylation. There were not significant changes between two groups in mRNAs levels of the methyl-binding domain (MBD) proteins, including MBD1 (Fig. 4D; P = 0.1195), MBD2 (Fig. 4E; P = 0.8887) or MBD4 (Fig. 4F; P = 0.1837) which could confer a higher affinity for single methylated CpG site, as well as thymine DNA glycosylase (TDG) (Fig. 4G; P = 0.3158).
Neonatal exposure to sevoflurane for three times decreases mRNA levels of the TET family in the hippocampus of mice at P37. RT-PCR analysis does not indicate significant changes of mRNA levels of DNA methyltransferases (DNMTs) including DNMT1 A. DNMT3a B. or DNMT3b C. methyl-binding proteins including MBD1 D. MBD2 E. or MBD4 F. or DNA demethylases TDG G. in hippocampus of the P37 mice undergoing sevoflurane anesthesia. However, RT-PCR analysis indicates significant reduction of mRNA levels of DNA hydroxymethylase including TET1 H. TET2 I. and TET3 J. in hippocampus of the P37 mice undergoing undergoing sevoflurane anesthesia. N = 5 per arm. Data were expressed as Mean ± SD. Student’s t-test. *P < 0.05, **P < 0.01. DNMT, DNA methyltransferases. MBD, methyl-CpG-binding domain. TDG, thymine DNA glycosylase. TET, ten-eleven translocation
However, there were significant reductions in mRNA levels of the ten-eleven translocation (TET) enzymes, including TET1 (Fig. 4H; 100% ± 25% vs. 63% ± 19%, P = 0.0281), TET2 (Fig. 4I; 100% ± 19% vs. 66% ± 18%, P = 0.0173) and TET3 (Fig. 4J; 100% ± 10% vs. 77% ± 11%, P = 0.0089). The TET enzymes are charging for hydroxymethylation of 5-methylcytosine (5mC) to form 5-hydroxymethylcytosine (5hmC). We performed immunobloting analysis to determine protein levels of the TET enzymes, and found that sevoflurane anesthesia at P6-8 was able to decrease protein levels of the TET3 ((Fig. 5E and F, Control vs. Sevo × 3; 100% ± 33% vs. 54% ± 23%, P = 0.0332 Student’s t-test), but not TET1 (Fig. 5A and B; 100% ± 60% vs. 119% ± 74%, P = 0.6631) or TET2 (Fig. 5C and D; 100% ± 26% vs. 135% ± 47%, P = 0.2379) in mouse hippocampus. Taken together, these findings suggested that sevoflurane anesthesia for three times in neonatal period could decrease the Tet mRNA levels and reduce the TET3 proteins, which could strengthen DNA methylation.
Neonatal exposure to sevoflurane for three times reduces protein levels of the TET3 in hippocampus of the P37 mice. A. Western blot analysis shows that sevoflurane anesthesia at P6-8 does not alter protein levels of the TET1 in hippocampus of the P37 mice, as compared with the control condition (N = 5 per group). There is no significant difference in the amounts of GAPDH in hippocampus of the mice following sevoflurane anesthesia or control condition. B. Quantification of immunoblotting shows that sevoflurane anesthesia does not alter hippocampal TET1 protein levels. C. Western blot analysis shows that sevoflurane anesthesia at P6-8 does not alter protein levels of the TET2 in hippocampus of the P37 mice, as compared with the control condition (N = 4 per group). There is no significant difference in the amounts of GAPDH in hippocampus of the mice following sevoflurane anesthesia or control condition. D. Quantification of immunoblotting shows that sevoflurane anesthesia does not alter hippocampal TET2 protein levels. E. Western blot analysis shows that sevoflurane anesthesia in neonatal period decreases protein levels of the TET3 in hippocampus of the mice as compared with the control condition (N = 5 per group). There is no significant difference in the amounts of GAPDH in hippocampus of the mice following sevoflurane anesthesia or control condition. F. Quantification of immunoblotting shows that sevoflurane anesthesia significantly decreases TET3 protein levels in mouse hippocampus. Data are expressed as Mean ± SD. Student’s t-test. *P < 0.05
4 Discussion
In this study, we found impairments of social recognition, learning and memory in the sevoflurane-anesthetized male mice. We demonstrated sevoflurane could reduce protein levels of SHANK2 and excitability of hippocampal neurons. In addition, we detected DNA hyper-methylation in the Shank2 gene. Collectively, these findings proved neonatal anesthesia with sevoflurane could induce developmental neurotoxicity and cognitive dysfunction in mice, with the mechanism of disturbing physiological DNA methylation in the Shank2 gene and reducing the SHANK2 protein. Moreover, we detected reduced mRNA and protein levels of the TET3 enzyme, which might be associated with silencing of the Shank2 gene.
Clinical trials reported that sevoflurane anesthesia in early infancy could not increase the risk of adverse neurodevelopmental outcomes at two and five years of age [22, 23]. A further investigation disclosed that these infants were anesthetized for ≤ 1 h and determined by Intelligence Quotient (IQ) scores at five-year-old, which were little appreciated due to the mild intensity of anesthesia and biased criterion of judgment. Sun et al. claimed that although a single anesthesia exposure before 36 months could not change IQ scores in later childhood, repeated and prolonged exposures are needed in future studies [24]. Moreover, the Mayo Anesthesia Safety in Kids (MASK) study reported that multiple, but not single, exposures to anesthesia could disturb executive function, behavior and reading in children [25]. Our current study showed neonatal exposures to sevoflurane for three times could disturb preference for social novelty in the P30 male mice. All these studies suggested the intricate phenotypes of sevoflurane-induced neurotoxicity, calling for more studies on the vulnerable subgroups [24] and reliable assessments [25, 26].
RNA-sequencing analysis indicated the Shank2 gene is strongly associated with learning, memory and social behaviors. In current study, the P30 mice showed abnormal social memory in recognizing the already-familiar Stranger 1 vs. the newly-introduced Stranger 2. Meanwhile, these mice showed normal social affiliation with conspecific mouse vs. inanimate empty enclosure. The P31-36 mice showed impairment of learning (i.e., increased escape latency) and memory (i.e., decreased platform-crossing times). The deletion or mutations of Shank/ProSAP genes were associated with neurodevelopmental disorders, including ASD, intellectual disability, and schizophrenia [27], which was in support of our findings.
The Shank genes are coding for postsynaptic scaffold proteins that are located in postsynaptic density (PSD) of the glutamatergic synapse [28]. The SHANK proteins are composed of five protein–protein interaction motifs, including 5–6 ankyrin repeated domains (ANK), a Src Homology 3 (SH3), a PDZ domain, a proline-rich region (Pro) and a sterile alpha motif (SAM) domain [28]. Leblond et al. reported that disruptions in the Shank gene family account for nearly 1% of all patients with autistic-like behavior [29]. Naisbitt et al. described that NMDA-type glutamate receptors are linked to PDZ domain of the SHANK proteins by GKAP/SAPAPs proteins, which bind to PSD95-NMDAR complex [30]. In previous studies, we found decreased NMDAR1 levels in hippocampus of the neonatal mice undergoing sevoflurane anesthesia plus abdominal surgery [31], and decreased PSD95 levels in hippocampus of the neonatal mice undergoing sevoflurane anesthesia [13]. Our present study showed decreased levels of the SHANK2 proteins in hippocampus of the P37 mice following sevoflurane anesthesia. Taken together, these studies suggested consistent alternations of SHANK2, PSD95 and NMDAR1. Although we detected reduced protein levels of the SHANK2 in mouse hippocampus, there were not significant changes in mRNA levels of the Shank1 and Shank3 genes. We have no idea of differential expressions of the Shank gene subtypes at present. One explanation might be that each Shank gene has a process-dynamic and domain-specific distribution.
It was reported that heterozygous loss-of-function mutations in Shank2 gene are associated with autism spectrum disorder [32]. In particular, PDZ domain of the Shank2 gene, with mutations of exons 16–17 and exons 17, are responsible for abnormal social behavior and impaired learning and memory [28], which was consistent with our findings of neurobehavioral outcomes and decreased SHANK2 protein levels. Multiple epigenetic mechanisms are cooperated to activate or suppress gene expression, which are important for normal neuronal development and function [11]. DNA hypermethylation is able to suppress gene expression, which is also regulated by diverse enzymes charging for DNA methylases. DNA methyltransferases are able to catalyze transfer of a methyl group from S-adenyl methionine to cytosine residue to form 5-methylcytosine (5mC). Proteins containing a conserved methyl-CpG-binding domain (MBD) show a higher affinity for single methylated CpG sites. Thymine DNA glycosylase (TDG) is able to replace the modified residue with a naked cytosine. Ten-eleven translocation (TET) enzymes are responsible for chemical modification of hydroxymethylation by adding a hydroxyl group onto the methyl group of 5mC to form 5hmC. In current study, sevoflurane anesthesia decreased mRNAs levels of the Tet genes, and reduced protein levels of the TET3 enzyme, which might be related to DNA hypermethylation of the Shank2 gene. The significance of the SHANK2-PSD95-NMDAR complex and the pathway of glutamatergic synapse make the SHANK2 protein become a potential target for attenuating sevoflurane-induced behavioral abnormalities in the neurodevelopmental disorders.
This study has several limitations. Firstly, we found deficit of social recognition memory and impairments of learning and memory in the mice following 3.0% sevoflurane plus 60% oxygen at P6-8, but we could not rule out influences of either 60% oxygen or repeated maternal deprivations [33, 34]. Our future studies will include the mice treated with 2 h separation from dams in air condition. Secondly, we analyzed expression of the Shank2 gene and DNA methylation-related enzymes merely in mouse hippocampus, but not in other brain regions like medial prefrontal cortex [35, 36]. After all, the hippocampus is most essential for learning, memory and cognition [20]. Thirdly, we did not conduct experiment to demonstrate the causality between the TET3 enzyme and the SHANK2 protein. However, the current findings had established a positive correlation between SHANK2 and TET3, and set an example for detecting upstream mechanism of reduced SHANK2 by investigating DNA methylation homeostasis, which could trigger more future studies on sevoflurane-induced developmental neurotoxicity in the pattern of epigenetic alternations.
5 Conclusions
In conclusion, neonatal anesthesia with sevoflurane for three times could induce neurobehavioral abnormalities in the male mice. DNA hypermethylation in the Shank2 gene could reduce synaptic scaffold protein SHANK2 and inhibit neuronal excitability in glutamatergic synapse, which could be explained by the reduced DNA hydroxymethylase TET3 (Fig. 6). The Shank2 gene could serve as a target gene for attenuating anesthetic-induced neurotoxicity in the developing brain, pending more future studies.
Availability of data and materials
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- WGBS:
-
Whole genome bisulfite sequencing
- DMRs:
-
Differentially methylated regions
- KEGG:
-
Kyoto encyclopedia of genes and genomes
- CG:
-
Cytosine-guanine
- RT-PCR:
-
Reverse transcription-polymerase chain reaction
- mRNA:
-
Messenger ribose nucleic acid
- PSD95:
-
Postsynaptic density protein 95
- NMDAR:
-
N-methyl-D-aspartic acid receptor
- TET:
-
Ten-eleven translocation
- GABA:
-
Gamma-amino butyric acid
- ASD:
-
Autism spectrum disorders
- GKAP:
-
Guanylate kinase-associated protein
- SAPAPs:
-
SAP90-associated proteins
- MWM:
-
Morris water maze
- cDNA:
-
Complementary deoxyribonucleic acid
- CT:
-
Cycle time
- GAPDH:
-
Glyceraldehyde-phosphate dehydrogenase
- NMDG:
-
N-Methyl-D-glucamine
- HEPES:
-
4-(2-Hydroxyethyl)-1-piperazineethanesulfonic acid
- sEPSCs:
-
Spontaneous excitatory postsynaptic currents
- IQR:
-
Interquartile range
- ANOVA:
-
Analysis of variance
- 5mC:
-
5-Methylcytosine
- 5hmC:
-
5-Hydroxymethylcytosine
- GO:
-
Gene ontology
- DNMT:
-
DNA methyltransferases
- MBD:
-
Methyl-CpG-binding domain
- TDG:
-
Thymine DNA glycosylase
- IQ:
-
Intelligence quotient
- MASK:
-
Mayo anesthesia safety in kids
- SAM:
-
Sterile alpha motif
- AAV:
-
Adeno-associated virus
References
Shi Y, Hu D, Rodgers EL, Katusic SK, Gleich SJ, Hanson AC, et al. Epidemiology of general anesthesia prior to age 3 in a population-based birth cohort. Paediatr Anaesth. 2018;28:513–9.
Vinson AE, Houck CS. Neurotoxicity of anesthesia in children: prevention and treatment. Curr Treat Options Neurol. 2018;20:51.
Vutskits L, Xie Z. Lasting impact of general anaesthesia on the brain: mechanisms and relevance. Nat Rev Neurosci. 2016;17:705–17.
Zhao X, Jin Y, Li H, Jia Y, Wang Y. Sevoflurane impairs learning and memory of the developing brain through post-transcriptional inhibition of CCNA2 via microRNA-19–3p. Aging (Albany NY). 2018;10(12):3794–805.
Zhang L, Zhang J, Yang L, Dong Y, Zhang Y, Xie Z. Isoflurane and sevoflurane increase interleukin-6 levels through the nuclear factor-kappa B pathway in neuroglioma cells. Br J Anaesth. 2013;110:i82–91.
Xie H, She G-M, Wang C, Zhang L-Y, Liu C-F. The gender difference in effect of sevoflurane exposure on cognitive function and hippocampus neuronal apoptosis in rats. Eur Rev Med Pharmacol Sci. 2015;19:647–57.
Dong Y, Zhang G, Zhang B, Moir RD, Xia W, Marcantonio ER, et al. The Common Inhalational Anesthetic Sevoflurane Induces Apoptosis and Increases β-Amyloid Protein Levels. Arch Neurol. 2009;66:620–31.
Fehr T, Janssen WGM, Park J, Baxter MG. Neonatal exposures to sevoflurane in rhesus monkeys alter synaptic ultrastructure in later life. Science. 2022;25:105685.
Xu G, Lu H, Dong Y, Shapoval D, Soriano SG, Liu X, et al. Coenzyme Q10 reduces sevoflurane-induced cognitive deficiency in young mice. Br J Anaesth. 2017;119:481–91.
Alam R, Abdolmaleky HM, Zhou J-R. Microbiome, inflammation, epigenetic alterations, and mental diseases. Am J Med Genet B Neuropsychiatr Genet. 2017;174:651–60.
Moore LD, Le T, Fan G. DNA methylation and its basic function. Neuropsychopharmacology. 2013;38:23–38.
Sala C, Vicidomini C, Bigi I, Mossa A, Verpelli C. Shank synaptic scaffold proteins: keys to understanding the pathogenesis of autism and other synaptic disorders. J Neurochem. 2015;135:849–58.
Liu H, Meng X, Li Y, Chen S, Ji Y, Song S, et al. Neonatal exposure to sevoflurane impairs preference for social novelty in C57BL/6 female mice at early-adulthood. Biochem Biophys Res Commun. 2022;593:129–36.
Lu H, Liufu N, Dong Y, Xu G, Zhang Y, Shu L, et al. Sevoflurane Acts on Ubiquitination-Proteasome Pathway to Reduce Postsynaptic Density 95 Protein Levels in Young Mice. Anesthesiology. 2017;127:961–75.
Chen Q, Chu W, Sheng R, Song S, Yang J, Ji F, et al. Maternal anesthesia with sevoflurane during the mid-gestation induces social interaction deficits in offspring C57BL/6 mice. Biochem Biophys Res Commun. 2021;553:65–71.
Zhao W, Song S, Chu W, Li Y, Chen S, Ji Y, et al. Disruption of hippocampal P2RX2/CaMKII/NF-kappaB signaling contributes to learning and memory impairment in C57BL/6 mice induced by surgery plus anesthesia in neonatal period. Biomed Pharmacother. 2022;149: 112897.
Zhang PA, Xu QY, Xue L, Zheng H, Yan J, Xiao Y, et al. Neonatal maternal deprivation enhances presynaptic P2X7 receptor transmission in insular cortex in an adult rat model of visceral hypersensitivity. CNS Neurosci Ther. 2017;23:145–54.
Li Y-C, Tian Y-Q, Wu Y-Y, Xu Y-C, Zhang P-A, Sha J, et al. Upregulation of Spinal ASIC1 and NKCC1 Expression Contributes to Chronic Visceral Pain in Rats. Front Mol Neurosci. 2021;13:611179.
Cheng Q, Song SH, Augustine GJ. Molecular mechanisms of short-term plasticity: role of synapsin phosphorylation in augmentation and potentiation of spontaneous glutamate release. Front Synaptic Neurosci. 2018;10:33.
Zeidman P, Maguire EA. Anterior hippocampus: the anatomy of perception, imagination and episodic memory. Nat Rev Neurosci. 2016;17:173–82.
Song S-Y, Meng X-W, Xia Z, Liu H, Zhang J, Chen Q-C, et al. Cognitiveimpairment and transcriptomic profile in hippocampus of young mice after multiple neonatal exposures to sevoflurane. Aging (Albany NY). 2019;11(19):8386–417.
Davidson AJ, Disma N, de Graaff JC, Withington DE, Dorris L, Bell G, et al. Neurodevelopmental outcome at 2 years of age after general anaesthesia and awake-regional anaesthesia in infancy (GAS): an international multicentre, randomised controlled trial. Lancet. 2016;387:239–50.
McCann ME, de Graaff JC, Dorris L, Disma N, Withington D, Bell G, et al. Neurodevelopmental outcome at 5 years of age after general anaesthesia or awake-regional anaesthesia in infancy (GAS): an international, multicentre, randomised, controlled equivalence trial. Lancet. 2019;393:664–77.
Sun LS, Li G, Miller TL, Salorio C, Byrne MW, Bellinger DC, et al. Association between a single general anesthesia exposure before age 36 months and neurocognitive outcomes in later childhood. JAMA. 2016;315:2312–20.
Warner DO, Zaccariello MJ, Katusic SK, Schroeder DR, Hanson AC, Schulte PJ, et al. Neuropsychological and behavioral outcomes after exposure of young children to procedures requiring general anesthesia: The Mayo Anesthesia Safety in Kids (MASK) study. Anesthesiology. 2018;129:89–105.
Makaryus R, Lee H, Robinson J, Enikolopov G, Benveniste H. Noninvasive tracking of anesthesia neurotoxicity in the developing rodent brain. Anesthesiology. 2018;129:118–30.
Mossa A, Giona F, Pagano J, Sala C, Verpelli C. SHANK genes in autism: defining therapeutic targets. Prog Neuropsychopharmacol Biol Psychiatry. 2018;84:416–23.
Monteiro P, Feng G. SHANK proteins: roles at the synapse and in autism spectrum disorder. Nat Rev Neurosci. 2017;18:147–57.
Leblond CS, Nava C, Polge A, Gauthier J, Huguet G, Lumbroso S, et al. Meta-analysis of SHANK mutations in autism spectrum disorders: a gradient of severity in cognitive impairments. PLoS Genet. 2014;10:e1004580.
Naisbitt S, Kim E, Tu JC, Xiao B, Sala C, Valtschanoff J, et al. Shank, a novel family of postsynaptic density proteins that binds to the NMDA receptor/PSD-95/GKAP complex and cortactin. Neuron. 1999;23:569–82.
Jin X, Ji L, Chen Q, Sheng R, Ji F, Yan J. Anesthesia plus surgery in neonatal period impairs preference for social novelty in mice at the juvenile age. Biochem Biophys Res Commun. 2020;530:603–8.
Zaslavsky K, Zhang WB, McCready FP, Rodrigues DC, Deneault E, Loo C, et al. SHANK2 mutations associated with autism spectrum disorder cause hyperconnectivity of human neurons. Nat Neurosci. 2019;22:556–64.
Wei F, Xian D, He Y, Yan Z, Deng X, Chen Y, et al. Effects of maternal deprivation and environmental enrichment on anxiety-like and depression-like behaviors correlate with oxytocin system and CRH level in the medial-lateral habenula. Peptides. 2022;158:170882.
Talani G, Biggio F, Gorule AA, Licheri V, Saolini E, Colombo D, et al. Sex-dependent changes of hippocampal synaptic plasticity and cognitive performance in C57BL/6J mice exposed to neonatal repeated maternal separation. Neuropharmacology. 2023;222:109301.
Xie L, Liu Y, Hu Y, Wang B, Zhu Z, Jiang Y, et al. Neonatal sevoflurane exposure induces impulsive behavioral deficit through disrupting excitatory neurons in the medial prefrontal cortex in mice. Transl Psychiatry. 2020;10:202.
Song R, Ling X, Peng M, Xue Z, Cang J, Fang F. Maternal sevoflurane exposure causes abnormal development of fetal prefrontal cortex and induces cognitive dysfunction in offspring. Stem Cells Int. 2017;2017:6158468.
Acknowledgements
We are grateful to Guangzhou Genedenovo Biotechnology Co., Ltd for assisting in WGBS analysis and bioinformatics analysis.
Funding
This work was supported by grants from National Natural Science Foundation of China (82001126 to S.Y.S).
Author information
Authors and Affiliations
Contributions
S-Y Song designed and performed the experiments, and analyzed the data. W-M Zhao and Y-M Ji performed the experiments, analyzed the data and prepared the figures. Q-H Huang, Y-X Li and S-W Chen performed the experiments and prepared the figures. J-P Yang supervised the experiments and revised the manuscript. X Jin designed the experiments, analyzed the data, and wrote the manuscript. The author(s) read and approved the final manuscript.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
The animal study was reviewed and approved by Institutional Animal Care and Use Committee of Soochow University.
Consent for publication
All authors listed above approved the submission of final manuscript.
Competing interests
The authors declare no conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Song, S., Zhao, W., Ji, Y. et al. SHANK2 protein contributes to sevoflurane-induced developmental neurotoxicity and cognitive dysfunction in C57BL/6 male mice. APS 1, 2 (2023). https://doi.org/10.1007/s44254-023-00005-7
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s44254-023-00005-7