Abstract
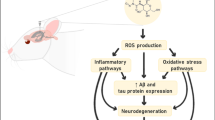
Brown adipose tissue (BAT) is a special type of fat tissue in mammals and is also a key endocrine organ in the human body. Batokine, the endocrine effector of BAT, plays a neuroprotective role and improves the prognosis by exerting anti-apoptotic and anti-inflammatory effects, as well as by improving vascular endothelial function and other mechanisms in nerve injury diseases. The present article briefly reviewed several types of batokines related to central nervous system (CNS) diseases. Following this, the potential therapeutic value and future research direction of batokines for CNS diseases were chiefly discussed from the aspects of protective mechanism and signaling pathway.



Similar content being viewed by others
Data Availability
Not applicable.
References
Betz MJ, Enerbäck S (2015) Human brown adipose tissue: what we have learned so far. Diabetes 64:2352–2360. https://doi.org/10.2337/db15-0146
Pinckard KM, Stanford KI (2021) The heartwarming effect of brown adipose Tissue. Mol Pharmacol 102:39–50. https://doi.org/10.1124/molpharm.121.000328
Cannon B, Nedergaard J (2004) Brown adipose tissue: function and physiological significance. Physiological Reviews 84:277–359. https://doi.org/10.1152/physrev.00015.2003
Nedergaard J, Bengtsson T, Cannon B (2007) Unexpected evidence for active brown adipose tissue in adult humans. Am J Physiol-Endocrinol Metab 293:E444–E452. https://doi.org/10.1152/ajpendo.00691.2006
van Marken Lichtenbelt WD, Vanhommerig JW, Smulders NM et al (2009) Cold-activated brown adipose tissue in healthy men. N Engl J Med 360:1500–1508. https://doi.org/10.1056/nejmoa0808718
Cypess AM, Lehman S, Williams G et al (2009) Identification and importance of brown adipose tissue in adult humans. N Engl J Med 360:1509–1517. https://doi.org/10.1056/nejmoa0810780
Villarroya F, Vidal-Puig A (2013) Beyond the sympathetic tone: the new brown fat activators. Cell Metabolism 17:638–643. https://doi.org/10.1016/j.cmet.2013.02.020
Scheele C, Wolfrum C (2019) Brown adipose crosstalk in tissue plasticity and human metabolism. Endocr Rev 41:53–65. https://doi.org/10.1210/endrev/bnz007
Townsend KL, Tseng Y-H (2015) Of mice and men: novel insights regarding constitutive and recruitable brown adipocytes. Int J Obes Supp 5:S15–S20. https://doi.org/10.1038/ijosup.2015.5
Nishio M, Saeki K (2020) The remaining mysteries about brown adipose tissues. Cells 9:2449. https://doi.org/10.3390/cells9112449
Lehnig AC, Dewal RS, Baer LA, et al (2019) Exercise training induces depot-specific adaptations to white and brown adipose tissue. iScience 11:425–439.https://doi.org/10.1016/j.isci.2018.12.033
Cannon B, Jong JMA, Fischer AW et al (2020) Human brown adipose tissue: classical brown rather than brite/beige? Exp Physiol 105:1191–1200. https://doi.org/10.1113/ep087875
de Jong JMA, Sun W, Pires ND et al (2019) Human brown adipose tissue is phenocopied by classical brown adipose tissue in physiologically humanized mice. Nat Metab 1:830–843. https://doi.org/10.1038/s42255-019-0101-4
Villarroya F, Cereijo R, Villarroya J, Giralt M (2016) Brown adipose tissue as a secretory organ. Nat Rev Endocrinol 13:26–35. https://doi.org/10.1038/nrendo.2016.136
Kita S, Maeda N, Shimomura I (2019) Interorgan communication by exosomes, adipose tissue, and adiponectin in metabolic syndrome. J Clin Invest 129:4041–4049. https://doi.org/10.1172/jci129193
Gavaldà-Navarro A, Villarroya J, Cereijo R et al (2021) The endocrine role of brown adipose tissue: an update on actors and actions. Rev Endocr Metab Disord. https://doi.org/10.1007/s11154-021-09640-6
Fon Tacer K, Bookout AL, Ding X et al (2010) Research resource: comprehensive expression atlas of the fibroblast growth factor system in adult mouse. Mol Endocrinol 24:2050–2064. https://doi.org/10.1210/me.2010-0142
Rydén M (2009) Fibroblast growth factor 21: an overview from a clinical perspective. Cell Mol Life Sci 66:2067–2073. https://doi.org/10.1007/s00018-009-0003-9
Xie T, Leung PS (2017) Fibroblast growth factor 21: a regulator of metabolic disease and health span. Am J Physiol-Endocrinol Metab 313:E292–E302. https://doi.org/10.1152/ajpendo.00101.2017
Kharitonenkov A (2009) FGFs and metabolism. Curr Opin Pharmacol 9:805–810. https://doi.org/10.1016/j.coph.2009.07.001
Tan BK, Hallschmid M, Adya R et al (2011) Fibroblast growth factor 21 (FGF21) in human cerebrospinal fluid. Diabetes 60:2758–2762. https://doi.org/10.2337/db11-0672
Hsuchou H, Pan W, Kastin AJ (2007) The fasting polypeptide FGF21 can enter brain from blood. Peptides 28:2382–2386. https://doi.org/10.1016/j.peptides.2007.10.007
Kharitonenkov A, Wroblewski VJ, Koester A et al (2007) The metabolic state of diabetic monkeys is regulated by fibroblast growth factor-21. Endocrinology 148:774–781. https://doi.org/10.1210/en.2006-1168
Lin Z, Pan X, Wu F et al (2015) Fibroblast growth factor 21 prevents atherosclerosis by suppression of hepatic sterol regulatory element-binding protein-2 and induction of adiponectin in mice. Circulation 131:1861–1871. https://doi.org/10.1161/circulationaha.115.015308
Yan X, Chen J, Zhang C et al (2015) FGF 21 deletion exacerbates diabetic cardiomyopathy by aggravating cardiac lipid accumulation. J Cell Mol Med 19:1557–1568. https://doi.org/10.1111/jcmm.12530
Yu Y, Bai F, Wang W et al (2015) Fibroblast growth factor 21 protects mouse brain against d-galactose induced aging via suppression of oxidative stress response and advanced glycation end products formation. Pharmacol Biochem Behav 133:122–131. https://doi.org/10.1016/j.pbb.2015.03.020
Huang X, Hu J, Li Y et al (2013) The cell adhesion molecule L1 regulates the expression of FGF21 and enhances neurite outgrowth. Brain Res 1530:13–21. https://doi.org/10.1016/j.brainres.2013.07.043
Sa-nguanmoo P, Chattipakorn N, Chattipakorn SC (2016) Potential roles of fibroblast growth factor 21 in the brain. Metab Brain Dis 31:239–248. https://doi.org/10.1007/s11011-015-9789-3
Sideromenos S, Gundacker A, Nikou M et al (2022) Uncoupling protein-1 modulates anxiety-like behavior in a temperature-dependent manner. J Neurosci 42:7659–7672. https://doi.org/10.1523/jneurosci.2509-21.2022
Yang X, Hui Q, Yu B et al (2022) Correction to “Design and evaluation of lyophilized fibroblast growth factor 21 and its protection against ischemia cerebral injury.”. Bioconjugate Chem 33:1437–1438. https://doi.org/10.1021/acs.bioconjchem.2c00234
Kuroda M, Muramatsu R, Maedera N et al (2017) Peripherally derived FGF21 promotes remyelination in the central nervous system. J Clin Invest 127:3496–3509. https://doi.org/10.1172/jci94337
Li S, Yu Y, Li L et al (2015) Treatment of CIA mice with FGF21 down-regulates TH17-IL-17 axis. Inflammation 39:309–319. https://doi.org/10.1007/s10753-015-0251-9
Hui X, Feng T, Liu Q et al (2016) The FGF21–adiponectin axis in controlling energy and vascular homeostasis. J Mol Cell Biol 8:110–119. https://doi.org/10.1093/jmcb/mjw013
Gómez-Sámano MÁ, Grajales-Gómez M, Zuarth-Vázquez JM et al (2017) Fibroblast growth factor 21 and its novel association with oxidative stress. Redox Biology 11:335–341. https://doi.org/10.1016/j.redox.2016.12.024
Wang N, Li J, Li S et al (2018) Fibroblast growth factor 21 regulates foam cells formation and inflammatory response in Ox-LDL-induced THP-1 macrophages. Biomed Pharmacother 108:1825–1834. https://doi.org/10.1016/j.biopha.2018.09.143
Tanajak P, Chattipakorn SC, Chattipakorn N (2015) Effects of fibroblast growth factor 21 on the heart. J Endocrinol 227:R13–R30. https://doi.org/10.1530/joe-15-0289
Xie Z, Dong X et al (2019) HMGB1-triggered inflammation inhibition of notoginseng leaf triterpenes against cerebral ischemia and reperfusion injury via MAPK and NF-κB signaling pathways. Biomolecules 9:512. https://doi.org/10.3390/biom9100512
Moskowitz MA, Lo EH, Iadecola C (2010) The science of stroke: mechanisms in search of treatments. Neuron 68:161. https://doi.org/10.1016/j.neuron.2010.08.019
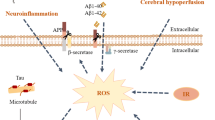
Wang D, Liu F, Zhu L et al (2020) FGF21 alleviates neuroinflammation following ischemic stroke by modulating the temporal and spatial dynamics of microglia/macrophages. J Neuroinflammation 17(1):257. https://doi.org/10.1186/s12974-020-01921-2
Tang Y, Le W (2015) Differential roles of M1 and M2 microglia in neurodegenerative diseases. Mol Neurobiol 53:1181–1194. https://doi.org/10.1007/s12035-014-9070-5
Ma Y, Wang J, Wang Y, Yang G-Y (2017) The biphasic function of microglia in ischemic stroke. Prog Neurobiol 157:247–272. https://doi.org/10.1016/j.pneurobio.2016.01.005
Bookout AL, de Groot MHM, Owen BM et al (2013) FGF21 regulates metabolism and circadian behavior by acting on the nervous system. Nat Med 19:1147–1152. https://doi.org/10.1038/nm.3249
Kurosu H, Choi M, Ogawa Y et al (2007) Tissue-specific expression of βKlotho and fibroblast growth factor (FGF) receptor isoforms determines metabolic activity of FGF19 and FGF21. J Biol Chem 282:26687–26695. https://doi.org/10.1074/jbc.m704165200
Adams AC, Cheng CC, Coskun T, Kharitonenkov A (2012) FGF21 requires βklotho to act in vivo. PLoS One 7:e49977. https://doi.org/10.1371/journal.pone.0049977
Itoh N, Ornitz DM (2010) Fibroblast growth factors: from molecular evolution to roles in development, metabolism and disease. J Biochem 149:121–130. https://doi.org/10.1093/jb/mvq121
Chen J, Hu J, Liu H et al (2018) FGF21 protects the blood–brain barrier by upregulating PPARγ via FGFR1/β-klotho after traumatic brain injury. J Neurotrauma 35:2091–2103. https://doi.org/10.1089/neu.2017.5271
Jiang Y, Liu N, Wang Q et al (2018) Endocrine regulator rFGF21 (recombinant human fibroblast growth factor 21) improves neurological outcomes following focal ischemic stroke of type 2 diabetes mellitus male mice. Stroke 49:3039–3049. https://doi.org/10.1161/strokeaha.118.022119
Pan J, Jin J, Ge H et al (2015) Malibatol A regulates microglia M1/M2 polarization in experimental stroke in a PPARγ-dependent manner. J Neuroinflammation 14(12):51. https://doi.org/10.1186/s12974-015-0270-3
Wang H-W, Jiang X, Zhang Y et al (2019) FGF21 protects against hypoxia injury through inducing HSP72 in cerebral microvascular endothelial cells. Front Pharmacol 10. https://doi.org/10.3389/fphar.2019.00101
Xiong T, Tang J, Zhao J et al (2012) Involvement of the Akt/GSK-3β/CRMP-2 pathway in axonal injury after hypoxic–ischemic brain damage in neonatal rat. Neuroscience 216:123–132. https://doi.org/10.1016/j.neuroscience.2012.04.052
Zhang W, Liu J, Hu X et al (2015) n -3 Polyunsaturated fatty acids reduce neonatal hypoxic/ischemic brain injury by promoting phosphatidylserine formation and akt signaling. Stroke 46:2943–2950. https://doi.org/10.1161/strokeaha.115.010815
Pan Y, Wang B, Zheng J et al (2018) Pancreatic fibroblast growth factor 21 protects against type 2 diabetes in mice by promoting insulin expression and secretion in a PI3K/Akt signaling-dependent manner. J Cell Mol Med 23:1059–1071. https://doi.org/10.1111/jcmm.14007
Lin MT, Beal MF (2006) Mitochondrial dysfunction and oxidative stress in neurodegenerative diseases. Nature 443:787–795. https://doi.org/10.1038/nature05292
Ballatore C, Lee VM-Y, Trojanowski JQ (2007) Tau-mediated neurodegeneration in Alzheimer’s disease and related disorders. Nat Rev Neurosci 8:663–672. https://doi.org/10.1038/nrn2194
Grundke-Iqbal I, Iqbal K, Tung Y-C et al (1987) Abnormal phosphorylation of the microtubule-associated protein? (tau) in Alzheimer cytoskeletal pathology. Alzheimer Dis Assoc Disord 1:202. https://doi.org/10.1097/00002093-198701030-00020
Long JM, Holtzman DM (2019) Alzheimer disease: an update on pathobiology and treatment strategies. Cell 179:312–339. https://doi.org/10.1016/j.cell.2019.09.001
Bélanger M, Allaman I, Magistretti PJ (2011) Brain energy metabolism: focus on astrocyte-neuron metabolic cooperation. Cell Metab 14:724–738. https://doi.org/10.1016/j.cmet.2011.08.016
Garden GA, La Spada AR (2012) Intercellular (mis)communication in neurodegenerative disease. Neuron 73:886–901. https://doi.org/10.1016/j.neuron.2012.02.017
Escartin C, Valette J, Lebon V, Bonvento G (2006) Neuron?astrocyte interactions in the regulation of brain energy metabolism: a focus on NMR spectroscopy. J Neurochem 99:393–401. https://doi.org/10.1111/j.1471-4159.2006.04083.x
Sun Y, Wang Y, Chen S-T et al (2020) Modulation of the astrocyte-neuron lactate shuttle system contributes to neuroprotective action of fibroblast growth factor 21. Theranostics 10:8430–8445. https://doi.org/10.7150/thno.44370
Sa-nguanmoo P, Tanajak P, Kerdphoo S et al (2018) FGF21 and DPP-4 inhibitor equally prevents cognitive decline in obese rats. Biomed Pharmacother 97:1663–1672. https://doi.org/10.1016/j.biopha.2017.12.021
Sa-nguanmoo P, Tanajak P, Kerdphoo S et al (2016) FGF21 improves cognition by restored synaptic plasticity, dendritic spine density, brain mitochondrial function and cell apoptosis in obese-insulin resistant male rats. Horm Behav 85:86–95. https://doi.org/10.1016/j.yhbeh.2016.08.006
Mäkelä J, Tselykh TV, Maiorana F et al (2014) Fibroblast growth factor-21 enhances mitochondrial functions and increases the activity of PGC-1α in human dopaminergic neurons via Sirtuin-1. SpringerPlus 3:2. https://doi.org/10.1186/2193-1801-3-2
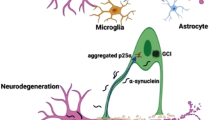
Yang C, Wang W, Deng P et al (2021) Fibroblast growth factor 21 modulates microglial polarization that attenuates neurodegeneration in mice and cellular models of Parkinson’s disease. Front Aging Neurosci 13. https://doi.org/10.3389/fnagi.2021.778527
Liddelow SA, Guttenplan KA, Clarke LE et al (2017) Neurotoxic reactive astrocytes are induced by activated microglia. Nature 541:481–487. https://doi.org/10.1038/nature21029
Qin Y, Qiu J, Wang P et al (2021) Impaired autophagy in microglia aggravates dopaminergic neurodegeneration by regulating NLRP3 inflammasome activation in experimental models of Parkinson’s disease. Brain Behav Immun 91:324–338. https://doi.org/10.1016/j.bbi.2020.10.010
Wang Q, Yuan J, Yu Z et al (2017) FGF21 attenuates high-fat diet-induced cognitive impairment via metabolic regulation and anti-inflammation of obese mice. Mol Neurobiol 55:4702–4717. https://doi.org/10.1007/s12035-017-0663-7
Campderrós L, Moure R, Cairó M et al (2019) Brown adipocytes secrete GDF15 in response to thermogenic activation. Obesity 27:1606–1616. https://doi.org/10.1002/oby.22584
Strelau J, Sullivan A, Böttner M et al (2000) Growth/differentiation factor-15/macrophage inhibitory cytokine-1 is a novel trophic factor for midbrain dopaminergic neurons in vivo. J Neurosci 20:8597–8603. https://doi.org/10.1523/jneurosci.20-23-08597.2000
Schober A, Böttner M, Strelau J et al (2001) Expression of growth differentiation factor-15/macrophage inhibitory cytokine-1 (GDF-15/MIC-1) in the perinatal, adult, and injured rat brain. J Comp Neurol 439:32–45. https://doi.org/10.1002/cne.1333
Fuchs T, Trollor JN, Crawford J et al (2013) Macrophage inhibitory cytokine-1 is associated with cognitive impairment and predicts cognitive decline - the Sydney memory and aging study. Aging Cell 12:882–889. https://doi.org/10.1111/acel.12116
Mu Y, Gage FH (2011) Adult hippocampal neurogenesis and its role in Alzheimer’s disease. Mol Neurodegener 6:85. https://doi.org/10.1186/1750-1326-6-85
Emmerson PJ, Wang F, Du Y et al (2017) The metabolic effects of GDF15 are mediated by the orphan receptor GFRAL. Nat Med 23:1215–1219. https://doi.org/10.1038/nm.4393
Yang L, Chang C-C, Sun Z et al (2017) GFRAL is the receptor for GDF15 and is required for the anti-obesity effects of the ligand. Nat Med 23:1158–1166. https://doi.org/10.1038/nm.4394
Li Z, Wang B, Wu X et al (2005) Identification, expression and functional characterization of the GRAL gene. J Neurochem 95:361–376. https://doi.org/10.1111/j.1471-4159.2005.03372.x
Tan M, Wang Y, Guan K, Sun Y (2000) PTGF-β , a type β transforming growth factor (TGF-β) superfamily member, is a p53 target gene that inhibits tumor cell growth via TGF-β signaling pathway. Proc Natl Acad Sci USA 97:109–114. https://doi.org/10.1073/pnas.97.1.109
Pagel J-I, Deindl E (2012) Disease progression mediated by Egr-1 associated signaling in response to oxidative stress. IJMS 13:13104–13117. https://doi.org/10.3390/ijms131013104
Kadowaki M, Yoshioka H, Kamitani H et al (2011) DNA methylation-mediated silencing of nonsteroidal anti-inflammatory drug-activated gene (NAG-1/GDF15) in glioma cell lines. Int J Cancer 130:267–277. https://doi.org/10.1002/ijc.26082
Woo SM, Min K, Kim S et al (2014) Silibinin induces apoptosis of HT29 colon carcinoma cells through early growth response-1 (EGR-1)-mediated non-steroidal anti-inflammatory drug-activated gene-1 (NAG-1) up-regulation. Chem-Biol Interact 211:36–43. https://doi.org/10.1016/j.cbi.2014.01.004
Yamaguchi H, Nishiyama M, Tokumoto S et al (2021) Elevated cytokine, chemokine, and growth and differentiation factor-15 levels in hemorrhagic shock and encephalopathy syndrome: a retrospective observational study. Cytokine 137:155324. https://doi.org/10.1016/j.cyto.2020.155324
Zhu S, Yang N, Guan Y et al (2021) GDF15 promotes glioma stem cell-like phenotype via regulation of ERK1/2–c-Fos–LIF signaling. Cell Death Discov 7. https://doi.org/10.1038/s41420-020-00395-8
Conte M, Martucci M, Chiariello A et al (2020) Mitochondria, immunosenescence and inflammaging: a role for mitokines? Semin Immunopathol 42:607–617. https://doi.org/10.1007/s00281-020-00813-0
Rochette L, Zeller M, Cottin Y, Vergely C (2020) Insights into mechanisms of GDF15 and receptor GFRAL: therapeutic targets. Trends Endocrinol Metab 31:939–951. https://doi.org/10.1016/j.tem.2020.10.004
Jin Y-J, Lee J-H, Kim Y-M et al (2012) Macrophage inhibitory cytokine-1 stimulates proliferation of human umbilical vein endothelial cells by up-regulating cyclins D1 and E through the PI3K/Akt-, ERK-, and JNK-dependent AP-1 and E2F activation signaling pathways. Cell Signal 24:1485–1495. https://doi.org/10.1016/j.cellsig.2012.03.014
Schindowski K, von Bohlen und Halbach O, Strelau J et al (2010) Regulation of GDF-15, a distant TGF-β superfamily member, in a mouse model of cerebral ischemia. Cell Tissue Res 343:399–409. https://doi.org/10.1007/s00441-010-1090-5
Song H, Yin D, Liu Z (2011) GDF-15 promotes angiogenesis through modulating p53/HIF-1α signaling pathway in hypoxic human umbilical vein endothelial cells. Mol Biol Rep 39:4017–4022. https://doi.org/10.1007/s11033-011-1182-7
Park H, Nam K-S, Lee H-J, Kim KS (2022) Ionizing radiation-induced GDF15 promotes angiogenesis in human glioblastoma models by promoting VEGFA expression through p-MAPK1/SP1 signaling. Front Oncol 12. https://doi.org/10.3389/fonc.2022.801230
Li M, Song K, Huang X et al (2018) GDF-15 prevents LPS and D-galactosamine-induced inflammation and acute liver injury in mice. Int J Mol Med. https://doi.org/10.3892/ijmm.2018.3747
Yuan L, Li S, Chen Q et al (2022) EBV infection-induced GPX4 promotes chemoresistance and tumor progression in nasopharyngeal carcinoma. Cell Death Differ 29:1513–1527. https://doi.org/10.1038/s41418-022-00939-8
Hou K, Shen J, Yan J et al (2021) Loss of TRIM21 alleviates cardiotoxicity by suppressing ferroptosis induced by the chemotherapeutic agent doxorubicin. EBioMedicine 69:103456. https://doi.org/10.1016/j.ebiom.2021.103456
Xia M, Zhang Q, Zhang Y et al (2022) Growth differentiation factor 15 regulates oxidative stress-dependent ferroptosis post spinal cord injury by stabilizing the p62-Keap1-Nrf2 signaling pathway. Front Aging Neurosci 14. https://doi.org/10.3389/fnagi.2022.905115
Zhang Y, Khan S, Liu Y et al (2022) Modes of brain cell death following intracerebral hemorrhage. Front Cell Neurosci 16. https://doi.org/10.3389/fncel.2022.799753
Chen J, Peng H, Chen C et al (2022) NAG-1/GDF15 inhibits diabetic nephropathy via inhibiting AGE/RAGE-mediated inflammation signaling pathways in C57BL/6 mice and HK-2 cells. Life Sci 311:121142. https://doi.org/10.1016/j.lfs.2022.121142
Brenière C, Méloux A, Pédard M et al (2019) Growth differentiation factor-15 (GDF-15) is associated with mortality in ischemic stroke patients treated with acute revascularization therapy. Front Neurol 10. https://doi.org/10.3389/fneur.2019.00611
Zang Y, Zhu Z, Xie Y et al (2022) Serum growth differentiation factor 15 levels are associated with depression after ischemic stroke. JAHA 11. https://doi.org/10.1161/jaha.121.022607
Worth AA, Shoop R, Tye K et al (2020) The cytokine GDF15 signals through a population of brainstem cholecystokinin neurons to mediate anorectic signalling. eLife 9. https://doi.org/10.7554/elife.55164
Cimino I, Kim H, Tung YCL et al (2021) Activation of the hypothalamic–pituitary–adrenal axis by exogenous and endogenous GDF15. Proc Natl Acad Sci USA 118. https://doi.org/10.1073/pnas.2106868118
Liu D-D, Lu J-M, Zhao Q-R et al (2016) Growth differentiation factor-15 promotes glutamate release in medial prefrontal cortex of mice through upregulation of T-type calcium channels. Sci Rep 6. https://doi.org/10.1038/srep28653
Wang Y, Zhen C, Wang R, Wang G (2019) Growth-differentiation factor-15 predicts adverse cardiac events in patients with acute coronary syndrome: a meta-analysis. Am J Emerg Med. https://doi.org/10.1016/j.ajem.2019.04.035
Xiong W-P, Yao W-Q, Wang B, Liu K (2021) BMSCs-exosomes containing GDF-15 alleviated SH-SY5Y cell injury model of Alzheimer’s disease via AKT/GSK-3β/β-catenin. Brain Res Bull 177:92–102. https://doi.org/10.1016/j.brainresbull.2021.09.008
Park B-N, Kim J-H, Lim TS et al (2020) Therapeutic effect of mesenchymal stem cells in an animal model of Alzheimer’s disease evaluated by β-amyloid positron emission tomography imaging. Aust N Z J Psychiatry 54:883–891. https://doi.org/10.1177/0004867420917467
Maetzler W, Deleersnijder W, Hanssens V et al (2016) GDF15/MIC1 and MMP9 Cerebrospinal fluid levels in Parkinson’s disease and lewy body dementia. PLoS One 11:e0149349. https://doi.org/10.1371/journal.pone.0149349
Machado V, Haas SJ-P, von Bohlen und Halbach O et al (2016) Growth/differentiation factor-15 deficiency compromises dopaminergic neuron survival and microglial response in the 6-hydroxydopamine mouse model of Parkinson’s disease. Neurobiol Dis 88:1–15. https://doi.org/10.1016/j.nbd.2015.12.016
Machado V, Gilsbach R, Das R et al (2016) Gdf-15 deficiency does not alter vulnerability of nigrostriatal dopaminergic system in MPTP-intoxicated mice. Cell Tissue Res 365:209–223. https://doi.org/10.1007/s00441-016-2406-x
Obeso JA, Rodriguez-Oroz MC, Goetz CG et al (2010) Missing pieces in the Parkinson’s disease puzzle. Nat Med 16:653–661. https://doi.org/10.1038/nm.2165
Ruth M (2012) A PGC1–α–dependent myokine that drives brown–fat–like development of white fat and thermogenesis. Yearbook of Endocrinol 2012:114–116. https://doi.org/10.1016/j.yend.2012.04.012
Yu Q, Kou W, Xu X et al (2019) FNDC5/Irisin inhibits pathological cardiac hypertrophy. Clin Sci 133:611–627. https://doi.org/10.1042/cs20190016
Tanhaei S, Nikpour P, Ghaedi K et al (2018) RNA/protein discordant expression of Fndc5 in central nervous system is likely to be mediated through microRNAs. DNA Cell Biol 37:373–380. https://doi.org/10.1089/dna.2017.4067
Wrann CD, White JP, Salogiannnis J et al (2013) Exercise induces hippocampal BDNF through a PGC-1α/FNDC5 pathway. Cell Metab 18:649–659. https://doi.org/10.1016/j.cmet.2013.09.008
Torma F, Bori Z, Koltai E et al (2014) Eating habits modulate short term memory and epigenetical regulation of brain derived neurotrophic factor in hippocampus of low- and high running capacity rats. Brain Res Bull 107:54–60. https://doi.org/10.1016/j.brainresbull.2014.07.003
Siteneski A, Olescowicz G, Pazini FL et al (2020) Antidepressant-like and pro-neurogenic effects of physical exercise: the putative role of FNDC5/irisin pathway. J Neural Transm 127:355–370. https://doi.org/10.1007/s00702-020-02143-9
Qin L, Bouchard R, Pugazhenthi S (2016) Regulation of cyclic AMP response element-binding protein during neuroglial interactions. J Neurochem 136:918–930. https://doi.org/10.1111/jnc.13497
Lourenco MV, Ribeiro FC, Sudo FK, et al (2020) Cerebrospinal fluid irisin correlates with amyloid-β, BDNF, and cognition in Alzheimer’s disease. Alzheimer’s & Dementia: Diagnosis, Assessment & Disease Monitoring 12. https://doi.org/10.1002/dad2.12034
Islam MR, Valaris S, Young MF et al (2021) Exercise hormone irisin is a critical regulator of cognitive function. Nat Metab 3:1058–1070. https://doi.org/10.1038/s42255-021-00438-z
Jodeiri Farshbaf M, Alviña K (2021) Multiple roles in neuroprotection for the exercise derived myokine irisin. Front Aging Neurosci 13. https://doi.org/10.3389/fnagi.2021.649929
Rabiee F, Lachinani L, Ghaedi S et al (2020) New insights into the cellular activities of Fndc5/Irisin and its signaling pathways. Cell Biosci 10. https://doi.org/10.1186/s13578-020-00413-3
Guo P, Liu L, Yang X et al (2022) Irisin improves BBB dysfunction in SAP rats by inhibiting MMP-9 via the ERK/NF-κB signaling pathway. Cell Signal 93:110300. https://doi.org/10.1016/j.cellsig.2022.110300
Zhao R (2022) Irisin at the crossroads of inter-organ communications: challenge and implications. Front Endocrinol 13. https://doi.org/10.3389/fendo.2022.989135
Ahmadi Ghahrizjani F, Ghaedi K, Salamian A et al (2015) Enhanced expression of FNDC5 in human embryonic stem cell-derived neural cells along with relevant embryonic neural tissues. Gene 557:123–129. https://doi.org/10.1016/j.gene.2014.12.010
Wang Y, Xu E, Musich PR, Lin F (2019) Mitochondrial dysfunction in neurodegenerative diseases and the potential countermeasure. CNS Neurosci Ther 25:816–824. https://doi.org/10.1111/cns.13116
Mani S, Swargiary G, Chadha R (2021) Mitophagy impairment in neurodegenerative diseases: pathogenesis and therapeutic interventions. Mitochondrion 57:270–293. https://doi.org/10.1016/j.mito.2021.01.001
Norris GT, Kipnis J (2018) Immune cells and CNS physiology: microglia and beyond. J Exp Med 216:60–70. https://doi.org/10.1084/jem.20180199
Gelders G, Baekelandt V, Van der Perren A (2018) Linking neuroinflammation and neurodegeneration in Parkinson’s disease. J Immunol Res 2018:1–12. https://doi.org/10.1155/2018/4784268
Qi J, Yang L, Wang X et al (2022) Irisin: a promising treatment for neurodegenerative diseases. Neuroscience 498:289–299. https://doi.org/10.1016/j.neuroscience.2022.07.018
Lourenco MV, de Freitas GB, Raony Í et al (2022) Irisin stimulates protective signaling pathways in rat hippocampal neurons. Front Cell Neurosci 16. https://doi.org/10.3389/fncel.2022.953991
Lourenco MV, Arancio O, Ferreira ST, De Felice FG (2019) P2-162: Exercise-linked FNDC5/Irisin corrects synaptic plasticity and memory defects in mouse models of Alzheimer’s disease. Alzheimer’s & Dementia 15:P637–P638. https://doi.org/10.1016/j.jalz.2019.06.2569
Noda Y, Kuzuya A, Tanigawa K et al (2018) Fibronectin type III domain-containing protein 5 interacts with APP and decreases amyloid β production in Alzheimer’s disease. Mol Brain 11. https://doi.org/10.1186/s13041-018-0401-8
Tu W-J, Qiu H-C, Cao J-L et al (2018) Decreased concentration of irisin is associated with poor functional outcome in ischemic stroke. Neurotherapeutics 15:1158–1167. https://doi.org/10.1007/s13311-018-0651-2
Tu T, Peng J, Jiang Y (2020) FNDC5/Irisin: a new protagonist in acute brain injury. Stem Cells Dev 29:533–543. https://doi.org/10.1089/scd.2019.0232
Øverberg LT, Lugg EF, Gaarder M et al (2022) Plasma levels of BDNF and EGF are reduced in acute stroke patients. Heliyon 8:e09661. https://doi.org/10.1016/j.heliyon.2022.e09661
Xu X, Zhou R, Ying J et al (2023) Irisin prevents hypoxic-ischemic brain damage in rats by inhibiting oxidative stress and protecting the blood-brain barrier. Peptides 161:170945. https://doi.org/10.1016/j.peptides.2023.170945
Jin Z, Zhang Z, Ke J et al (2021) Exercise-linked irisin prevents mortality and enhances cognition in a mice model of cerebral ischemia by regulating klotho expression. Oxid Med Cell Longevity 2021:1–16. https://doi.org/10.1155/2021/1697070
Jin Z, Guo P, Li X et al (2019) Neuroprotective effects of irisin against cerebral ischemia/reperfusion injury via Notch signaling pathway. Biomed Pharmacother 120:109452. https://doi.org/10.1016/j.biopha.2019.109452
Zhu D, Wang H, Zhang J et al (2015) Irisin improves endothelial function in type 2 diabetes through reducing oxidative/nitrative stresses. J Mol Cell Cardiol 87:138–147. https://doi.org/10.1016/j.yjmcc.2015.07.015
Lu J, Xiang G, Liu M et al (2015) Irisin protects against endothelial injury and ameliorates atherosclerosis in apolipoprotein E-Null diabetic mice. Atherosclerosis 243:438–448. https://doi.org/10.1016/j.atherosclerosis.2015.10.020
Wang Y, Tian M, Tan J et al (2022) Irisin ameliorates neuroinflammation and neuronal apoptosis through integrin αVβ5/AMPK signaling pathway after intracerebral hemorrhage in mice. J Neuroinflammation 19. https://doi.org/10.1186/s12974-022-02438-6
Peng J, Deng X, Huang W et al (2017) Irisin protects against neuronal injury induced by oxygen-glucose deprivation in part depends on the inhibition of ROS-NLRP3 inflammatory signaling pathway. Mol Immunol 91:185–194. https://doi.org/10.1016/j.molimm.2017.09.014
Yu Q, Li G, Ding Q et al (2020) Irisin protects brain against ischemia/reperfusion injury through suppressing TLR4/MyD88 pathway. Cerebrovasc Dis 49:346–354. https://doi.org/10.1159/000505961
Acknowledgements
Ming Shen and Min Zhang contributed equally to this work and should be considered co-first authors.
Funding
This study was funded by the National Natural Science Foundation of China (82271747).
Author information
Authors and Affiliations
Contributions
All authors Ming Shen, Min Zhang, Niping Mao, and Zhenlang Lin made a significant contribution to the work reported, whether that is in the conception, study design, execution, and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Corresponding author
Ethics declarations
Ethics Approval
Not applicable.
Consent to Participate
Not applicable.
Consent for Publication
All the authors read the manuscript carefully and gave their consent for publication.
Competing Interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Ming Shen and Min Zhang are co-first authors.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Shen, M., Zhang, M., Mao, N. et al. Batokine in Central Nervous System Diseases. Mol Neurobiol 60, 7021–7031 (2023). https://doi.org/10.1007/s12035-023-03490-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12035-023-03490-w