Abstract
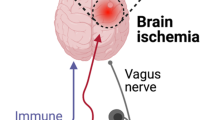
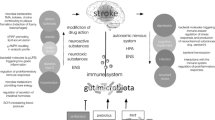
The gut microbiota are not only related to the development and occurrence of digestive system disease, but also have a bidirectional relationship with nervous system diseases via the microbiota-gut-brain axis. At present, correlations between the gut microbiota and neurological diseases, including stroke, are one of the focuses of investigation and attention in the medical community. Ischemic stroke (IS) is a cerebrovascular disease accompanied by focal neurological deficit or central nervous system injury or death. In this review, we summarize the contemporary latest research on correlations between the gut microbiota and IS. Additionally, we discuss the mechanisms of gut microbiota implicated in IS and related to metabolite production and immune regulation. Moreover, the factors of gut microbiota that affecting IS occurrence, and research implicating the gut microbiota as potential therapeutic targets for IS, are highlighted. Our review highlights the evidential relationships and connections between the gut microbiota and IS pathogenesis and prognosis.



Similar content being viewed by others
Data Availability
The datasets generated during and/or analyzed during the current study are available in the Pubmed repository, https://pubmed.ncbi.nlm.nih.gov/.
References
Elgendy IY, Mahmoud AN, Mansoor H et al (2016) Evolution of acute ischemic stroke therapy from lysis to thrombectomy: similar or different to acute myocardial infarction? Int J Cardiol 222:441–447. https://doi.org/10.1016/j.ijcard.2016.07.251
Johnson W, Onuma O, Owolabi M et al (2016) Stroke: a global response is needed. Bull World Health Organ 94(9):634-634A. https://doi.org/10.2471/BLT.16.181636
Yang G, Wang Y, Zeng Y et al (2013) Rapid health transition in China, 1990–2010: findings from the Global Burden of Disease Study 2010. Lancet 381(9882):1987–2015. https://doi.org/10.1016/S0140-6736(13)61097-1
Hathidara MY, Saini V, Malik AM (2019) Stroke in the young: a global update. Curr Neurol Neurosci Rep 19(11):91. https://doi.org/10.1007/s11910-019-1004-1
Marini C, Russo T, Felzani G (2010) Incidence of stroke in young adults: a review. Stroke Res Treat 2011:535672. https://doi.org/10.4061/2011/535672
Wang W, Jiang B, Sun H et al (2017) prevalence, incidence, and mortality of stroke in China: results from a nationwide population-based survey of 480 687 adults. Circulation 135(8):759–771. https://doi.org/10.1161/CIRCULATIONAHA.116.025250
Ahlawat S, Asha SKK (2021) Gut-organ axis: a microbial outreach and networking. Lett Appl Microbiol 72(6):636–668. https://doi.org/10.1111/lam.13333
Mayer EA, Nance K, Chen S (2022) The gut-brain axis. Annu Rev Med 73:439–453. https://doi.org/10.1146/annurev-med-042320-014032
Stilling RM, Dinan TG, Cryan JF (2014) Microbial genes, brain & behaviour-epigenetic regulation of the gut-brain axis. Genes Brain Behav 13(1):69–86. https://doi.org/10.1111/gbb.12109
Wilson ID, Nicholson JK (2017) Gut microbiome interactions with drug metabolism, efficacy, and toxicity. Transl Res 179:204–222. https://doi.org/10.1016/j.trsl.2016.08.002
Wang F, Ye Y, Xin C et al (2021) Candida albicans triggers qualitative and temporal responses in gut bacteria. J Mycol Med 31(3):101164. https://doi.org/10.1016/j.mycmed.2021.101164
Moon J, Yoon CH, Choi SH et al (2020) Can gut microbiota affect dry eye syndrome? Int J Mol Sci 21(22):8443. https://doi.org/10.3390/ijms21228443
Thursby E, Juge N (2017) Introduction to the human gut microbiota. Biochem J 474(11):1823–1836. https://doi.org/10.1042/BCJ20160510
Parker A, Fonseca S, Carding SR (2020) Gut microbes and metabolites as modulators of blood-brain barrier integrity and brain health. Gut Microbes 11(2):135–157. https://doi.org/10.1080/19490976.2019.1638722
Krautkramer KA, Fan J, Bäckhed F (2021) Gut microbial metabolites as multi-kingdom intermediates. Nat Rev Microbiol 19(2):77–94. https://doi.org/10.1038/s41579-020-0438-4
Cong J, Zhou P, Zhang R (2022) Intestinal microbiota-derived short chain fatty acids in host health and disease. Nutrients 14(9):1977. https://doi.org/10.3390/nu14091977
Janeiro MH, Ramírez MJ, Milagro FI et al (2018) Implication of trimethylamine n-Oxide (TMAO) in disease: potential biomarker or new therapeutic target. Nutrients 10(10):1398. https://doi.org/10.3390/nu10101398
Vascellari S, Palmas V, Melis M et al (2020) Gut microbiota and metabolome alterations associated with Parkinson’s disease. mSystems 5(5):e00561-20. https://doi.org/10.1128/mSystems.00561-20
Peh A, O’Donnell JA, Broughton BRS et al (2022) Gut microbiota and their metabolites in stroke: a double-edged sword. Stroke 53(5):1788–1801. https://doi.org/10.1161/STROKEAHA.121.036800
Li N, Wang X, Sun C et al (2019) Change of intestinal microbiota in cerebral ischemic stroke patients. BMC Microbiol 19(1):191. https://doi.org/10.1186/s12866-019-1552-1
Guo C, Huo YJ, Li Y et al (2022) Gut-brain axis: focus on gut metabolites short-chain fatty acids. World J Clin Cases 10(6):1754–1763. https://doi.org/10.12998/wjcc.v10.i6.1754
Khosravi A, Yáñez A, Price JG et al (2014) Gut microbiota promote hematopoiesis to control bacterial infection. Cell Host Microbe 15(3):374–81. https://doi.org/10.1016/j.chom.2014.02.006
O’Keefe SJ (2016) Diet, microorganisms and their metabolites, and colon cancer. Nat Rev Gastroenterol Hepatol 13(12):691–706. https://doi.org/10.1038/nrgastro.2016.165
Smolinska S, O’Mahony L (2016) Microbiome-host immune system interactions. Semin Liver Dis 36(4):317–326. https://doi.org/10.1055/s-0036-1593883
Zhu S, Jiang Y, Xu K et al (2020) The progress of gut microbiome research related to brain disorders. J Neuroinflammation 17(1):25. https://doi.org/10.1186/s12974-020-1705-z
Medzhitov R, Janeway C Jr (2000) Innate immune recognition: mechanisms and pathways. Immunol Rev 173:89–97. https://doi.org/10.1034/j.1600-065x.2000.917309.x
Honda K, Littman DR (2016) The microbiota in adaptive immune homeostasis and disease. Nature 535(7610):75–84. https://doi.org/10.1038/nature18848
Dinan TG, Cryan JF (2017) Gut instincts: microbiota as a key regulator of brain development, ageing and neurodegeneration. J Physiol 595(2):489–503. https://doi.org/10.1113/JP273106
Fung TC (2020) The microbiota-immune axis as a central mediator of gut-brain communication. Neurobiol Dis 136:104714. https://doi.org/10.1016/j.nbd.2019.104714
Kadowaki A, Quintana FJ (2020) The gut-CNS axis in multiple sclerosis. Trends Neurosci 43(8):622–634. https://doi.org/10.1016/j.tins.2020.06.002
Ganal SC, Sanos SL, Kallfass C et al (2012) Priming of natural killer cells by nonmucosal mononuclear phagocytes requires instructive signals from commensal microbiota. Immunity 37(1):171–186. https://doi.org/10.1016/j.immuni.2012.05.020
Park J, Kim M, Kang SG et al (2015) Short-chain fatty acids induce both effector and regulatory T cells by suppression of histone deacetylases and regulation of the mTOR-S6K pathway. Mucosal Immunol 8(1):80–93. https://doi.org/10.1038/mi.2014.44
Sadler R, Cramer JV, Heindl S et al (2020) Short-chain fatty acids improve poststroke recovery via immunological mechanisms. J Neurosci 40(5):1162–1173. https://doi.org/10.1523/JNEUROSCI.1359-19.2019
Zhang Z, Zhang H, Chen T et al (2022) Regulatory role of short-chain fatty acids in inflammatory bowel disease. Cell Commun Signal 20(1):64. https://doi.org/10.1186/s12964-022-00869-5
Machiels K, Joossens M, Sabino J et al (2014) A decrease of the butyrate-producing species roseburia hominis and Faecalibacterium prausnitzii defines dysbiosis in patients with ulcerative colitis. Gut 63(8):1275–1283. https://doi.org/10.1136/gutjnl-2013-304833
Li AL, Ni WW, Zhang QM et al (2020) Effect of cinnamon essential oil on gut microbiota in the mouse model of dextran sodium sulfate-induced colitis. Microbiol Immunol 64(1):23–32. https://doi.org/10.1111/1348-0421.12749
Mendelson SJ, Prabhakaran S (2021) Diagnosis and management of transient ischemic attack and acute ischemic stroke: a review. JAMA 325(11):1088–1098. https://doi.org/10.1001/jama.2020.26867
El-Koussy M, Schroth G, Brekenfeld C et al (2014) Imaging of acute ischemic stroke. Eur Neurol 72(5–6):309–316. https://doi.org/10.1159/000362719
Zhao Y, Zhang X, Chen X et al (2022) Neuronal injuries in cerebral infarction and ischemic stroke: from mechanisms to treatment (Review). Int J Mol Med 49(2):15. https://doi.org/10.3892/ijmm.2021.5070
Li JJ, Fang CH (2004) Atheroscleritis is a more rational term for the pathological entity currently known as atherosclerosis. Med Hypotheses 63(1):100–102. https://doi.org/10.1016/j.mehy.2004.01.029
Jin R, Liu L, Zhang S et al (2013) Role of inflammation and its mediators in acute ischemic stroke. J Cardiovasc Transl Res 6(5):834–851. https://doi.org/10.1007/s12265-013-9508-6
Krnjević K (1999) Early effects of hypoxia on brain cell function. Croat Med J 40(3):375–380
Jin W, Wu Y, Chen N et al (2021) Early administration of MPC-n(IVIg) selectively accumulates in ischemic areas to protect inflammation-induced brain damage from ischemic stroke. Theranostics 11(17):8197–8217. https://doi.org/10.7150/thno.58947
Martin RL, Lloyd HG, Cowan AI (1994) The early events of oxygen and glucose deprivation: setting the scene for neuronal death? Trends Neurosci 17(6):251–257. https://doi.org/10.1016/0166-2236(94)90008-6
Tuo QZ, Zhang ST, Lei P (2022) Mechanisms of neuronal cell death in ischemic stroke and their therapeutic implications. Med Res Rev 42(1):259–305. https://doi.org/10.1002/med.21817
Atlante A, Calissano P, Bobba A et al (2001) Glutamate neurotoxicity, oxidative stress and mitochondria. FEBS Lett 497(1):1–5. https://doi.org/10.1016/s0014-5793(01)02437-1
Jayaraj RL, Azimullah S, Beiram R et al (2019) Neuroinflammation: friend and foe for ischemic stroke. J Neuroinflammation 16(1):142. https://doi.org/10.1186/s12974-019-1516-2
Lucas SM, Rothwell NJ, Gibson RM (2006) The role of inflammation in CNS injury and disease. Br J Pharmacol 147(Suppl 1):S232-40. https://doi.org/10.1038/sj.bjp.0706400
Wang J, Xing H, Wan L et al (2018) Treatment targets for M2 microglia polarization in ischemic stroke. Biomed Pharmacother 105:518–525. https://doi.org/10.1016/j.biopha.2018.05.143
Latta CH, Sudduth TL, Weekman EM et al (2015) Determining the role of IL-4 induced neuroinflammation in microglial activity and amyloid-β using BV2 microglial cells and APP/PS1 transgenic mice. J Neuroinflammation 12:41. https://doi.org/10.1186/s12974-015-0243-6
Mecha M, Feliú A, Carrillo-Salinas FJ et al (2015) Endocannabinoids drive the acquisition of an alternative phenotype in microglia. Brain Behav Immun 49:233–245. https://doi.org/10.1016/j.bbi.2015.06.002
Lai AY, Todd KG (2006) Microglia in cerebral ischemia: molecular actions and interactions. Can J Physiol Pharmacol 84(1):49–59. https://doi.org/10.1139/Y05-143
Anrather J, Iadecola C (2016) Inflammation and stroke: an overview. Neurotherapeutics 13(4):661–670. https://doi.org/10.1007/s13311-016-0483-x
Liesz A, Suri-Payer E, Veltkamp C et al (2009) Regulatory T cells are key cerebroprotective immunomodulators in acute experimental stroke. Nat Med 15(2):192–199. https://doi.org/10.1038/nm.1927
Shi Y, Guo L, Chen Y et al (2021) Risk factors for ischemic stroke: differences between cerebral small vessel and large artery atherosclerosis aetiologies. Folia Neuropathol 59(4):378–385. https://doi.org/10.5114/fn.2021.112007
Karlsson FH, Fåk F, Nookaew I et al (2012) Symptomatic atherosclerosis is associated with an altered gut metagenome. Nat Commun 3:1245. https://doi.org/10.1038/ncomms2266
Jie Z, Xia H, Zhong SL et al (2017) The gut microbiome in atherosclerotic cardiovascular disease. Nat Commun 8(1):845. https://doi.org/10.1038/s41467-017-00900-1
Liu H, Chen X, Hu X et al (2019) Alterations in the gut microbiome and metabolism with coronary artery disease severity. Microbiome 7(1):68. https://doi.org/10.1186/s40168-019-0683-9
Qin J, Li Y, Cai Z et al (2012) A metagenome-wide association study of gut microbiota in type 2 diabetes. Nature 490(7418):55–60. https://doi.org/10.1038/nature11450
Li J, Zhang H, Wang G (2020) Correlations between inflammatory response, oxidative stress, intestinal pathological damage and intestinal flora variation in rats with type 2 diabetes mellitus. Eur Rev Med Pharmacol Sci 24(19):10162–10168. https://doi.org/10.26355/eurrev_202010_23236
Wu X, Ma C, Han L et al (2010) Molecular characterisation of the faecal microbiota in patients with type II diabetes. Curr Microbiol 61(1):69–78. https://doi.org/10.1007/s00284-010-9582-9
Zhang X, Shen D, Fang Z et al (2013) Human gut microbiota changes reveal the progression of glucose intolerance. PLoS One 8(8):e71108. https://doi.org/10.1371/journal.pone.0071108
Toral M, Robles-Vera I, de la Visitación N et al (2019) Critical role of the interaction gut microbiota - sympathetic nervous system in the regulation of blood pressure. Front Physiol 10:231. https://doi.org/10.3389/fphys.2019.00231
Mushtaq N, Hussain S, Zhang S et al (2019) Molecular characterization of alterations in the intestinal microbiota of patients with grade 3 hypertension. Int J Mol Med 44(2):513–522. https://doi.org/10.3892/ijmm.2019.4235
Ley RE, Bäckhed F, Turnbaugh P et al (2005) Obesity alters gut microbial ecology. Proc Natl Acad Sci U S A 102(31):11070–11075. https://doi.org/10.1073/pnas.0504978102
Karlsson FH, Tremaroli V, Nookaew I et al (2013) Gut metagenome in European women with normal, impaired and diabetic glucose control. Nature 498(7452):99–103. https://doi.org/10.1038/nature12198
Guo HH, Shen HR, Tang MZ et al (2023) Microbiota-derived short-chain fatty acids mediate the effects of dengzhan shengmai in ameliorating cerebral ischemia via the gut-brain axis. J Ethnopharmacol 306:116158. https://doi.org/10.1016/j.jep.2023.116158
Säemann MD, Böhmig GA, Osterreicher CH et al (2000) Anti-inflammatory effects of sodium butyrate on human monocytes: potent inhibition of IL-12 and up-regulation of IL-10 production. FASEB J 14(15):2380–2382. https://doi.org/10.1096/fj.00-0359fje
Zhou Z, Xu N, Matei N et al (2021) Sodium butyrate attenuated neuronal apoptosis via GPR41/Gβγ/PI3K/Akt pathway after MCAO in rats. J Cereb Blood Flow Metab 41(2):267–281. https://doi.org/10.1177/0271678X20910533
Margolis KG, Cryan JF, Mayer EA (2021) The microbiota-gut-brain axis: from motility to mood. Gastroenterology 160(5):1486–1501. https://doi.org/10.1053/j.gastro.2020.10.066
Sharma A, Castellani RJ, Smith MA et al (2019) 5-Hydroxytryptophan: a precursor of serotonin influences regional blood-brain barrier breakdown, cerebral blood flow, brain edema formation, and neuropathology. Int Rev Neurobiol 146:1–44. https://doi.org/10.1016/bs.irn.2019.06.005
Glebov K, Löchner M, Jabs R et al (2015) Serotonin stimulates secretion of exosomes from microglia cells. Glia 63(4):626–634. https://doi.org/10.1002/glia.22772
Yang W, Yu T, Huang X et al (2020) Intestinal microbiota-derived short-chain fatty acids regulation of immune cell IL-22 production and gut immunity. Nat Commun 11(1):4457. https://doi.org/10.1038/s41467-020-18262-6
Inoue D, Kimura I, Wakabayashi M et al (2012) Short-chain fatty acid receptor GPR41-mediated activation of sympathetic neurons involves synapsin 2b phosphorylation. FEBS Lett 586(10):1547–1554. https://doi.org/10.1016/j.febslet.2012.04.021
Karlsson C, Ahrné S, Molin G et al (2010) Probiotic therapy to men with incipient arteriosclerosis initiates increased bacterial diversity in colon: a randomized controlled trial. Atherosclerosis 208(1):228–233. https://doi.org/10.1016/j.atherosclerosis.2009.06.019
Yamashiro K, Kurita N, Urabe T et al (2021) Role of the gut microbiota in stroke pathogenesis and potential therapeutic implications. Ann Nutr Metab 77(Suppl 2):36–44. https://doi.org/10.1159/000516398
Tu R, Xia J (2023) Stroke and vascular cognitive impairment: the role of intestinal microbiota metabolite TMAO. CNS Neurol Disord Drug Targets. https://doi.org/10.2174/1871527322666230203140805
Zhang Y, Wang Y, Ke B et al (2021) TMAO: how gut microbiota contributes to heart failure. Transl Res 228:109–125. https://doi.org/10.1016/j.trsl.2020.08.007
Thomas MS, Fernandez ML (2021) Trimethylamine N-Oxide (TMAO), diet and cardiovascular disease. Curr Atheroscler Rep 23(4):12. https://doi.org/10.1007/s11883-021-00910-x
Zhu W, Gregory JC, Org E et al (2016) Gut microbial metabolite TMAO enhances platelet hyperreactivity and thrombosis risk. Cell 165(1):111–124. https://doi.org/10.1016/j.cell.2016.02.011
Svingen GFT, Zuo H, Ueland PM et al (2018) Increased plasma trimethylamine-N-oxide is associated with incident atrial fibrillation. Int J Cardiol 267:100–106. https://doi.org/10.1016/j.ijcard.2018.04.128
Jalife J (2014) Mechanisms of persistent atrial fibrillation. Curr Opin Cardiol 29(1):20–27. https://doi.org/10.1097/HCO.0000000000000027
Koeth RA, Wang Z, Levison BS et al (2013) Intestinal microbiota metabolism of L-carnitine, a nutrient in red meat, promotes atherosclerosis. Nat Med 19(5):576–585. https://doi.org/10.1038/nm.3145
Wang Z, Klipfell E, Bennett BJ et al (2011) Gut flora metabolism of phosphatidylcholine promotes cardiovascular disease. Nature 472(7341):57–63. https://doi.org/10.1038/nature09922
Klein DC, Skjesol A, Kers-Rebel ED et al (2015) CD14, TLR4 and TRAM show different trafficking dynamics during LPS stimulation. Traffic 16(7):677–690. https://doi.org/10.1111/tra.12274
Leblhuber F, Ehrlich D, Steiner K et al (2021) The immunopathogenesis of Alzheimer’s disease is related to the composition of gut microbiota. Nutrients 13(2):361. https://doi.org/10.3390/nu13020361
Logsdon AF, Erickson MA, Rhea EM et al (2018) Gut reactions: how the blood-brain barrier connects the microbiome and the brain. Exp Biol Med (Maywood) 243(2):159–165. https://doi.org/10.1177/1535370217743766
Benakis C, Brea D, Caballero S et al (2016) Commensal microbiota affects ischemic stroke outcome by regulating intestinal γδ T cells. Nat Med 22(5):516–523. https://doi.org/10.1038/nm.4068
Ridler C (2016) Gut microbiota: gut bacteria affect post-ischaemic inflammation in stroke by modulating intestinal T cells. Nat Rev Gastroenterol Hepatol 13(5):250. https://doi.org/10.1038/nrgastro.2016.64
Kurokawa S (2021) The link between gut microbiota and cerebral functions: an update on the pathogenesis and treatment of functional gastrointestinal disorders. Brain Nerve 73(8):857–862. https://doi.org/10.11477/mf.1416201852. Japanese
Wei W, Wang S, Xu C et al (2022) Gut microbiota, pathogenic proteins and neurodegenerative diseases. Front Microbiol 13:959856. https://doi.org/10.3389/fmicb.2022.959856
Winek K, Engel O, Koduah P et al (2016) Depletion of cultivatable gut microbiota by broad-spectrum antibiotic pretreatment worsens outcome after murine stroke. Stroke 47(5):1354–1363. https://doi.org/10.1161/STROKEAHA.115.011800
Doll DN, Engler-Chiurazzi EB, Lewis SE et al (2015) Lipopolysaccharide exacerbates infarct size and results in worsened post-stroke behavioral outcomes. Behav Brain Funct 11(1):32. https://doi.org/10.1186/s12993-015-0077-5
Crapser J, Ritzel R, Verma R et al (2016) Ischemic stroke induces gut permeability and enhances bacterial translocation leading to sepsis in aged mice. Aging (Albany NY) 8(5):1049–63. https://doi.org/10.18632/aging.100952
Caso JR, Hurtado O, Pereira MP et al (2009) Colonic bacterial translocation as a possible factor in stress-worsening experimental stroke outcome. Am J Physiol Regul Integr Comp Physiol 296(4):R979–R985. https://doi.org/10.1152/ajpregu.90825.2008
Lee J, d’Aigle J, Atadja L et al (2020) Gut microbiota-derived short-chain fatty acids promote poststroke recovery in aged mice. Circ Res 127(4):453–465. https://doi.org/10.1161/CIRCRESAHA.119.316448
Spychala MS, Venna VR, Jandzinski M et al (2018) Age-related changes in the gut microbiota influence systemic inflammation and stroke outcome. Ann Neurol 84(1):23–36. https://doi.org/10.1002/ana.25250
Tan C, Wu Q, Wang H et al (2021) Dysbiosis of gut microbiota and short-chain fatty acids in acute ischemic stroke and the subsequent risk for poor functional outcomes. JPEN J Parenter Enteral Nutr 45(3):518–529. https://doi.org/10.1002/jpen.1861
Kurita N, Yamashiro K, Kuroki T et al (2020) Metabolic endotoxemia promotes neuroinflammation after focal cerebral ischemia. J Cereb Blood Flow Metab 40(12):2505–2520. https://doi.org/10.1177/0271678X19899577
Singh V, Roth S, Llovera G et al (2016) Microbiota dysbiosis controls the neuroinflammatory response after stroke. J Neurosci 36(28):7428–7440. https://doi.org/10.1523/JNEUROSCI.1114-16.2016
Mahmoudi H, Hossainpour H (2023) Application and development of fecal microbiota transplantation in the treatment of gastrointestinal and metabolic diseases: a review. Saudi J Gastroenterol 29(1):3–11. https://doi.org/10.4103/sjg.sjg_131_22
Vendrik KEW, Ooijevaar RE, de Jong PRC et al (2020) Fecal microbiota transplantation in neurological disorders. Front Cell Infect Microbiol 10:98. https://doi.org/10.3389/fcimb.2020.00098
Chen R, Xu Y, Wu P et al (2019) Transplantation of fecal microbiota rich in short chain fatty acids and butyric acid treat cerebral ischemic stroke by regulating gut microbiota. Pharmacol Res 148:104403. https://doi.org/10.1016/j.phrs.2019.104403
Tiwari P, Dwivedi R, Bansal M et al (2023) Role of gut microbiota in neurological disorders and its therapeutic significance. J Clin Med 12(4):1650. https://doi.org/10.3390/jcm12041650
Derrien M, van Hylckama Vlieg JE (2015) Fate, activity, and impact of ingested bacteria within the human gut microbiota. Trends Microbiol 23(6):354–366. https://doi.org/10.1016/j.tim.2015.03.002
Wang Z, Xiao G, Yao Y et al (2006) The role of bifidobacteria in gut barrier function after thermal injury in rats. J Trauma 61(3):650–657. https://doi.org/10.1097/01.ta.0000196574.70614.27
Li JM, Yu R, Zhang LP et al (2019) Dietary fructose-induced gut dysbiosis promotes mouse hippocampal neuroinflammation: a benefit of short-chain fatty acids. Microbiome 7(1):98. https://doi.org/10.1186/s40168-019-0713-7
Parada Venegas D, De la Fuente MK, Landskron G et al (2019) Short chain fatty acids (SCFAs)-mediated gut epithelial and immune regulation and its relevance for inflammatory bowel diseases. Front Immunol 10:277. https://doi.org/10.3389/fimmu.2019.00277
Tang WH, Wang Z, Levison BS et al (2013) Intestinal microbial metabolism of phosphatidylcholine and cardiovascular risk. N Engl J Med 368(17):1575–1584. https://doi.org/10.1056/NEJMoa1109400
Meng X, Wang M, Wang X et al (2014) Suppression of NADPH oxidase- and mitochondrion-derived superoxide by Notoginsenoside R1 protects against cerebral ischemia-reperfusion injury through estrogen receptor-dependent activation of Akt/Nrf2 pathways. Free Radic Res 48(7):823–38. https://doi.org/10.3109/10715762.2014.911853
Li H, Xiao J, Li X et al (2018) Low cerebral exposure cannot hinder the neuroprotective effects of panax notoginsenosides. Drug Metab Dispos 46(1):53–65. https://doi.org/10.1124/dmd.117.078436
Huang XP, Ding H, Lu JD et al (2015) Effects of the combination of the main active components of astragalus and panax notoginseng on inflammation and apoptosis of nerve cell after cerebral ischemia-reperfusion. Am J Chin Med 43(7):1419–1438. https://doi.org/10.1142/S0192415X15500809
Chen R, Wu P, Cai Z et al (2019) Puerariae lobatae radix with chuanxiong rhizoma for treatment of cerebral ischemic stroke by remodeling gut microbiota to regulate the brain-gut barriers. J Nutr Biochem 65:101–114. https://doi.org/10.1016/j.jnutbio.2018.12.004
Li Y, Liang W, Guo C et al (2020) Renshen shouwu extract enhances neurogenesis and angiogenesis via inhibition of TLR4/NF-κB/NLRP3 signaling pathway following ischemic stroke in rats. J Ethnopharmacol 253:112616. https://doi.org/10.1016/j.jep.2020.112616
Wan L, Cheng Y, Luo Z et al (2015) Neuroprotection, learning and memory improvement of a standardized extract from renshen shouwu against neuronal injury and vascular dementia in rats with brain ischemia. J Ethnopharmacol 165:118–126. https://doi.org/10.1016/j.jep.2015.02.027
Funding
This research was supported financially by the Sichuan Science and Technology Program (2023NSFSC1698, 2022NSFSC1539, 2023NSFSC0529, and 2022YFS0629), Technology Strategic Cooperation Project of Luzhou Municipal People’s Government–Southwest Medical University (2018LZNYD-ZK26), and the Foundation of Southwest Medical University (2022QN042, 2022QN085, 2022QN102, and 2022QN118).
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. The first draft of the manuscript was written by Tao Wang and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Ethics Approval
Not applicable
Consent to Participate
Not applicable
Consent for Publication
Not applicable
Competing Interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Wang, T., Pan, C., Xie, C. et al. Microbiota Metabolites and Immune Regulation Affect Ischemic Stroke Occurrence, Development, and Prognosis. Mol Neurobiol 60, 6176–6187 (2023). https://doi.org/10.1007/s12035-023-03473-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12035-023-03473-x