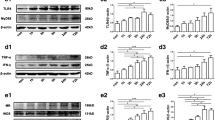
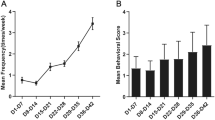
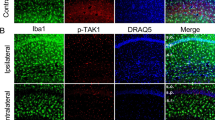
Abstract
Epilepsy is one of the most common neurological disorders. The pro-epileptic and antiepileptic roles of microglia have recently garnered significant attention. Interleukin-1 receptor-associated kinase (IRAK)-M, an important kinase in the innate immune response, is mainly expressed in microglia and acts as a negative regulator of the TLR4 signaling pathway that mediates the anti-inflammatory effect. However, whether IRAK-M exerts a protective role in epileptogenesis as well as the molecular and cellular mechanisms underlying these processes are yet to be elucidated. An epilepsy mouse model induced by pilocarpine was used in this study. Real-time quantitative polymerase chain reaction and western blot analysis were used to analyze mRNA and protein expression levels, respectively. Whole-cell voltage-clamp recordings were employed to evaluate the glutamatergic synaptic transmission in hippocampal neurons. Immunofluorescence was utilized to show the glial cell activation and neuronal loss. Furthermore, the proportion of microglia was analyzed using flow cytometry. Seizure dynamics influenced the expression of IRAK-M. Its knockout dramatically exacerbated the seizures and the pathology in epilepsy and increased the N-methyl-d-aspartate receptor (NMDAR) expression, thereby enhancing glutamatergic synaptic transmission in hippocampal CA1 pyramidal neurons in mice. Furthermore, IRAK-M deficiency augmented hippocampal neuronal loss via a possible mechanism of NMDAR-mediated excitotoxicity. IRAK-M deletion promotes microglia toward the M1 phenotype, which resulted in high levels of proinflammatory cytokines and was accompanied by a visible increase in the expressions of key microglial polarization-related proteins, including p-STAT1, TRAF6, and SOCS1. The findings demonstrate that IRAK-M dysfunction contributes to the progression of epilepsy by increasing M1 microglial polarization and glutamatergic synaptic transmission. This is possibly related to NMDARs, particularly Grin2A and Grin2B, which suggests that IRAK-M could serve as a novel therapeutic target for the direct alleviation of epilepsy.
Graphical Abstract








Similar content being viewed by others
Data Availability
Data is contained within the article. The data that support the findings of this study are available from the corresponding author upon reasonable request.
References
Riney K, Bogacz A, Somerville E, Hirsch E, Nabbout R, Scheffer IE et al (2022) International League Against Epilepsy classification and definition of epilepsy syndromes with onset at a variable age: position statement by the ILAE Task Force on Nosology and Definitions. Epilepsia 63(6):1443–1474
Thijs RD, Surges R, O’Brien TJ, Sander JW (2019) Epilepsy in adults. Lancet 393(10172):689–701
Rogawski MA, Loscher W (2004) The neurobiology of antiepileptic drugs. Nat Rev Neurosci 5(7):553–564
Kwan P, Schachter SC, Brodie MJ (2011) Drug-resistant epilepsy. N Engl J Med 365(10):919–926
Eyo UB, Murugan M, Wu LJ (2017) Microglia-neuron communication in epilepsy. Glia 65(1):5–18
Hu XM, Leak RK, Shi YJ, Suenaga J, Gao YQ, Zheng P et al (2015) Microglial and macrophage polarization -new prospects for brain repair. Nat Rev Neurol 11(1):56–64
Benson MJ, Manzanero S, Borges K (2015) Complex alterations in microglial M1/M2 markers during the development of epilepsy in two mouse models. Epilepsia 56(6):895–905
Boche D, Perry VH, Nicoll JAR (2013) Review: activation patterns of microglia and their identification in the human brain. Neuropath Appl Neuro 39(1):3–18
Cherry JD, Olschowka JA, O’Banion MK (2014) Neuroinflammation and M2 microglia: the good, the bad, and the inflamed. J Neuroinflammation 11:98
Hiragi T, Ikegaya Y, Koyama R (2018) Microglia after seizures and in epilepsy. Cells 7(4)
Kleen JK, Holmes GL (2010) Taming TLR4 may ease seizures. Nat Med 16(4):369–370
Liu L, Xu Y, Dai H, Tan S, Mao X, Chen Z (2020) Dynorphin activation of kappa opioid receptor promotes microglial polarization toward M2 phenotype via TLR4/NF-kappaB pathway. Cell Biosci 10:42
Yang WH, Li J, Shang Y, Zhao L, Wang MY, Shi JP et al (2017) HMGB1-TLR4 axis Plays a regulatory role in the pathogenesis of mesial temporal lobe epilepsy in immature rat model and children via the p38MAPK signaling pathway. Neurochem Res 42(4):1179–1190
Kamasak T, Dilber B, Yaman SO, Durgut BD, Kurt T, Coban E et al (2020) HMGB-1, TLR4, IL-1R1, TNF-alpha, and IL-1beta: novel epilepsy markers? Epileptic Disord 22(2):183–193
von Ruden EL, Gualtieri F, Schonhoff K, Reiber M, Wolf F, Baumgartner W et al (2020) Molecular alterations of the TLR4-signaling cascade in canine epilepsy. BMC Vet Res 16(1):18
Cunha C, Gomes C, Vaz AR, Brites D (2016) Exploring new inflammatory biomarkers and pathways during LPS-induced M1 polarization. Mediators Inflamm 2016:6986175
Janssens S, Beyaert R (2003) Functional diversity and regulation of different interleukin-1 receptor-associated kinase (IRAK) family members. Mol Cell 11(2):293–302
Kobayashi K, Hernandez LD, Galan JE, Janeway CA Jr, Medzhitov R, Flavell RA (2002) IRAK-M is a negative regulator of Toll-like receptor signaling. Cell 110(2):191–202
Lyu CF, Zhang YF, Gu MH, Huang YS, Liu GH, Wang C et al (2018) IRAK-M deficiency exacerbates ischemic neurovascular injuries in experimental stroke mice. Front Cell Neurosci 12
Liu B, Gu Y, Pei S, Peng Y, Chen J, Pham LV et al (2019) Interleukin-1 receptor associated kinase (IRAK)-M -mediated type 2 microglia polarization ameliorates the severity of experimental autoimmune encephalomyelitis (EAE). J Autoimmun 102:77–88
Deng N, Hu J, Hong Y, Ding Y, Xiong Y, Wu Z et al (2021) Indoleamine-2,3-dioxygenase 1 deficiency suppresses seizures in epilepsy. Front Cell Neurosci 15:638854
Racine RJ (1975) Modification of seizure activity by electrical stimulation: cortical areas. Electroencephalogr Clin Neurophysiol 38(1):1–12
Cortez MA, Perez Velazquez JL, Snead OC 3rd. (2006) Animal models of epilepsy and progressive effects of seizures. Adv Neurol 97:293–304
Li WP, Su XH, Hu NY, Hu J, Li XW, Yang JM et al (2022) Astrocytes mediate cholinergic regulation of adult hippocampal neurogenesis and memory through M1 muscarinic receptor. Biol Psychiatry
Su XH, Li WP, Wang YJ, Liu J, Liu JY, Jiang Y et al (2022) Chronic administration of 13-cis-retinoic acid induces depression-like behavior by altering the activity of dentate granule cells. Neurotherapeutics 19(1):421–433
Steibel J, Poletto R, Rosa G (2005) Statistical analysis of relative quantification of gene expression using real time RT-PCR data. J Anim Sci 83:104–104
Young K, Morrison H (2018) Quantifying microglia morphology from photomicrographs of immunohistochemistry prepared tissue using imageJ. J Vis Exp 136
Sun H, He JC, Wu JW, Zhan SQ, Zhang GL, Wu HQ et al (2019) Losartan inhibits development of spontaneous recurrent seizures by preventing astrocyte activation and attenuating blood-brain barrier permeability following pilocarpine-induced status epilepticus. Brain Res Bull 149:251–259
Wang N, Mi X, Gao B, Gu J, Wang W, Zhang Y et al (2015) Minocycline Inhibits brain inflammation and attenuates spontaneous recurrent seizures following pilocarpine-induced status epilepticus. Neuroscience 287:144–156
Amakhin DV, Malkin SL, Ergina JL, Kryukov KA, Veniaminova EA, Zubareva OE et al (2017) Alterations in properties of glutamatergic transmission in the temporal cortex and hippocampus following pilocarpine-induced acute seizures in wistar rats. Front Cell Neurosci 11:264
Goldberg EM, Coulter DA (2013) Mechanisms of epileptogenesis: a convergence on neural circuit dysfunction. Nat Rev Neurosci 14(5):337–349
Zhang W, Huguenard JR, Buckmaster PS (2012) Increased excitatory synaptic input to granule cells from hilar and CA3 regions in a rat model of temporal lobe epilepsy. J Neurosci 32(4):1183–1196
Devinsky O, Vezzani A, Najjar S, De Lanerolle NC, Rogawski MA (2013) Glia and epilepsy: excitability and inflammation. Trends Neurosci 36(3):174–184
Du J, Nicolaes GA, Kruijswijk D, Versloot M, van der Poll T, van ’t Veer C. (2014) The structure function of the death domain of human IRAK-M. Cell Commun Signal 12:77
Shen H, Goldstein DR (2009) IRAK-M is a negative regulator of inflammation that inhibits the graft prolonging effects of costimulatory blockade. Am J Transplant 9:255–255
Nimmerjahn A, Kirchhoff F, Helmchen F (2005) Resting microglial cells are highly dynamic surveillants of brain parenchyma in vivo. Science 308(5726):1314–1318
Butturini E, Boriero D, Carcereri de Prati A, Mariotto S (2019) STAT1 drives M1 microglia activation and neuroinflammation under hypoxia. Arch Biochem Biophys 669:22–30
Wu HJ, Zheng JW, Xu SB, Fang YJ, Wu YX, Zeng JX et al (2021) Mer regulates microglial/macrophage M1/M2 polarization and alleviates neuroinflammation following traumatic brain injury. J Neuroinflamm 18(1)
Curia G, Longo D, Biagini G, Jones RS, Avoli M (2008) The pilocarpine model of temporal lobe epilepsy. J Neurosci Methods 172(2):143–157
Levesque M, Biagini G, de Curtis M, Gnatkovsky V, Pitsch J, Wang SY et al (2021) The pilocarpine model of mesial temporal lobe epilepsy: over one decade later, with more rodent species and new investigative approaches. Neurosci Biobehav R 130:274–291
Ren E, Curia G (2021) Synaptic reshaping and neuronal outcomes in the temporal lobe epilepsy. Int J Mol Sci 22(8)
Delgado-Escueta AV, Bajorek JG (1982) Status epilepticus: mechanisms of brain damage and rational management. Epilepsia 23(Suppl 1):S29–S41
Tan Z, Sankar R, Shin D, Sun N, Liu H, Wasterlain CG et al (2002) Differential induction of p53 in immature and adult rat brain following lithium-pilocarpine status epilepticus. Brain Res 928(1-2):187–193
Simmons ML, Terman GW, Chavkin C (1997) Spontaneous excitatory currents and kappa-opioid receptor inhibition in dentate gyrus are increased in the rat pilocarpine model of temporal lobe epilepsy. J Neurophysiol 78(4):1860–1868
Zhan RZ, Timofeeva O, Nadler JV (2010) High Ratio of synaptic excitation to synaptic inhibition in hilar ectopic granule cells of pilocarpine-treated rats. J Neurophysiol 104(6):3293–3304
Zhou Y, Danbolt NC (2014) Glutamate as a neurotransmitter in the healthy brain. J Neural Transm 121(8):799–817
Lopes MW, Soares FMS, de Mello N, Nunes JC, Cajado AG, de Brito D et al (2013) Time-dependent modulation of AMPA receptor phosphorylation and mRNA expression of NMDA receptors and glial glutamate transporters in the rat hippocampus and cerebral cortex in a pilocarpine model of epilepsy. Exp Brain Res 226(2):153–163
Naylor DE, Liu HT, Niquet J, Wasterlain CG (2013) Rapid surface accumulation of NMDA receptors increases glutamatergic excitation during status epilepticus. Neurobiol Dis 54:225–238
Borges K, Gearing M, McDermott DL, Smith AB, Almonte AG, Wainer BH et al (2003) Neuronal and glial pathological changes during epileptogenesis in the mouse pilocarpine model. Exp Neurol 182(1):21–34
Shigemoto-Mogami Y, Hoshikawa K, Goldman JE, Sekino Y, Sato K (2014) Microglia enhance neurogenesis and oligodendrogenesis in the early postnatal subventricular zone. J Neurosci 34(6):2231–2243
Deng JC, Cheng G, Newstead MW, Zeng X, Kobayashi K, Flavell RA et al (2006) Sepsis-induced suppression of lung innate immunity is mediated by IRAK-M. J Clin Invest 116(9):2532–2542
Cianciulli A, Calvello R, Porro C, Trotta T, Panaro MA (2017) Understanding the role of SOCS signaling in neurodegenerative diseases: current and emerging concepts. Cytokine Growth Factor Rev 37:67–79
Croker BA, Kiu H, Nicholson SE (2008) SOCS regulation of the JAK/STAT signalling pathway. Semin Cell Dev Biol 19(4):414–422
Vezzani A, Balosso S, Ravizza T (2019) Neuroinflammatory pathways as treatment targets and biomarkers in epilepsy. Nat Rev Neurol 15(8):459–472
Quirico-Santos T, Meira ID, Gomes AC, Pereira VC, Pinto M, Monteiro M et al (2013) Resection of the epileptogenic lesion abolishes seizures and reduces inflammatory cytokines of patients with temporal lobe epilepsy. J Neuroimmunol 254(1-2):125–130
Strauss KI, Elisevich KV (2016) Brain region and epilepsy-associated differences in inflammatory mediator levels in medically refractory mesial temporal lobe epilepsy. J Neuroinflamm:13
Vezzani A, Viviani B (2015) Neuromodulatory properties of inflammatory cytokines and their impact on neuronal excitability. Neuropharmacology 96(Pt A):70–82
Fogal B, Hewett SJ (2008) Interleukin-1 beta: a bridge between inflammation and excitotoxicity? J Neurochem 106(1):1–23
Viviani B, Bartesaghi S, Gardoni F, Vezzani A, Behrens MM, Bartfai T et al (2003) Interleukin-1beta enhances NMDA receptor-mediated intracellular calcium increase through activation of the Src family of kinases. J Neurosci 23(25):8692–8700
Henstridge CM, Tzioras M, Paolicelli RC (2019) Glial contribution to excitatory and inhibitory synapse loss in neurodegeneration. Front Cell Neurosci 13:63
Iori V, Frigerio F, Vezzani A (2016) Modulation of neuronal excitability by immune mediators in epilepsy. Curr Opin Pharmacol 26:118–123
Acknowledgments
The authors would like to acknowledge Prof. Hong-Hao Wang from the Department of Neurology, Nanfang Hospital, Southern Medical University for presenting IRAK-M−/− mice, and to express sincere gratitude to the editor and anonymous reviewers for their valuable comments.
Funding
This work was supported by grants from the National Natural Science Foundation of China (No. 81873158, No. 82074265), the Natural Science Foundation of Guangdong Province, China (No.2021A1515011505, No.2020A1515010324), and Construction Fund of Key Disciplines of Traditional Chinese Medicine in Guangdong, China (No. G622299957).
Author information
Authors and Affiliations
Contributions
Wei Xie, Wei-Peng Li, Yue-Wen Ding, and Xiao-Shan Liang conceived and designed the experiments and revised the manuscript; Ting-Lin Qian, Yi-Fan Xiong, Xiao-Tao Liang, and Xiao-Yu Zhu performed the experiments; Yun-Lv Li, Jie-Li Zhou, and Le-Yi Tan assisted in some of the experimental work; Ting-Lin Qian and Wei-Peng Li analyzed data; The first draft of the manuscript was written by Xiao-Shan Liang, and all authors commented on previous versions of the manuscript. All authors have read and approved the final manuscript.
Corresponding authors
Ethics declarations
Ethics Approval
All animal experimental protocols in this study were approved by the Southern Medical University Institutional Animal Care and Use Committee (permit number: L2020038) and conducted in accordance with the National Health and Medical Research Council animal ethics and ARRIVE guidelines. The experimental mice were housed in a controlled temperature (23–25°C) and humidity (45–55%) with a modified 12-h dark-light photophase (lights on from 07:00 AM to 07:00 PM) and free access to standard food and water.
Consent to Participate
This is an animal experiment, with no human participants.
Consent for Publication
All co-authors have explicitly acknowledged receipt of the material and consented to its publication.
Competing interests
The authors declare no competing exists.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
ESM 1
(DOCX 2.94 MB)
(MP4 978 kb)
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Liang, XS., Qian, TL., Xiong, YF. et al. IRAK-M Ablation Promotes Status Epilepticus-Induced Neuroinflammation via Activating M1 Microglia and Impairing Excitatory Synaptic Function. Mol Neurobiol 60, 5199–5213 (2023). https://doi.org/10.1007/s12035-023-03407-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12035-023-03407-7