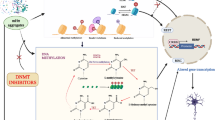
Abstract
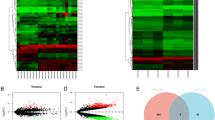
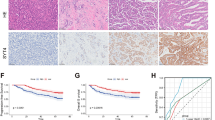
Chemotherapy-induced neuropathic pain (CINP) is a dose-limiting adverse event affecting 40% of chemotherapy patients. MiRNA-mRNA interaction plays an important role in various processes. However, detailed profiling of miRNA-mRNA interactions in CINP remains unclear. Here, a rat-based CINP model was established using paclitaxel, followed by nociceptive behavioral tests related to mechanical allodynia, thermal hyperalgesia, and cold allodynia. The landscape of miRNA-mRNA interaction in the spinal dorsal horn was investigated through mRNA transcriptomics and small RNA sequencing. Under CINP condition, 86 differentially expressed mRNAs and 56 miRNAs were identified. Gene Set Enrichment Analysis (GSEA), Gene Ontology (GO), and Kyoto Encyclopedia of Genes and Genomes (KEGG) pathway enrichment analyses indicated the activity of Odorant binding, postsynaptic specialization and synaptic density, extracellular matrix, mitochondrial matrix, retrograde endocannabinoid signaling, and GTPase activity. Protein–protein interaction (PPI), networks of circRNA-miRNA-mRNA, lncRNA-miRNA-mRNA, and TF-genes were demonstrated. We next explored the immune infiltration microenvironment and found a higher infiltration abundance of Th17 and a lower abundance of MDSC in CINP. RT-qPCR and dual-luciferase assays were used to verify the sequencing results, and single-cell analysis based on the SekSeeq database was conducted. Combined with bioinformatics analyses and experimental validations, Mpz, a protein-coding gene specifically expressed in Schwann cells, was found critical in maintaining CINP under miRNA regulation. Therefore, these data highlight the expression patterns of miRNA-mRNA, and the underlying mechanism in the spinal dorsal horn under CINP condition, and Mpz may serve as a promising therapeutic target for patients with CINP.








Similar content being viewed by others
Data Availability
Code and data that support the findings of this study are available from the corresponding author upon reasonable request.
References
Staff NP, Grisold A, Grisold W, Windebank AJ (2017) Chemotherapy-induced peripheral neuropathy: a current review. Ann Neurol 81(6):772–781. https://doi.org/10.1002/ana.24951
Gornstein E, Schwarz TL (2014) The paradox of paclitaxel neurotoxicity: mechanisms and unanswered questions. Neuropharmacology 76(Pt A):175–183. https://doi.org/10.1016/j.neuropharm.2013.08.016
Pease-Raissi SE, Pazyra-Murphy MF, Li Y, Wachter F, Fukuda Y, Fenstermacher SJ, Barclay LA, Bird GH, Walensky LD, Segal RA (2017) Paclitaxel reduces axonal bclw to initiate IP3R1-dependent axon degeneration. Neuron 96(2):373–38. https://doi.org/10.1016/j.neuron.2017.09.034
Zhang J, Pan Y, Shi Q, Zhang G, Jiang L, Dong X, Gu K, Wang H, Zhang X, Yang N, Li Y, Xiong J, Yi T, Peng M, Song Y, Fan Y, Cui J, Chen G, Tan W, Zang A, Guo Q, Zhao G, Wang Z, He J, Yao W, Wu X, Chen K, Hu X, Hu C, Yue L, Jiang D, Wang G, Liu J, Yu G, Li J, Bai J, Xie W, Zhao W, Wu L, Zhou C (2022) Paclitaxel liposome for injection (Lipusu) plus cisplatin versus gemcitabine plus cisplatin in the first-line treatment of locally advanced or metastatic lung squamous cell carcinoma: A multicenter, randomized, open-label, parallel controlled clinical study. Cancer Commun (Lond) 42(1):3–16. https://doi.org/10.1002/cac2.12225
Rodwin RL, Kairalla JA, Hibbitts E, Devidas M, Whitley MK, Mohrmann CE, Schore RJ, Raetz E et al (2022) Persistence of chemotherapy-induced peripheral neuropathy despite vincristine reduction in childhood B-acute lymphoblastic leukemia. J Natl Cancer Inst 114(8):1167–1175. https://doi.org/10.1093/jnci/djac095
Mezzanotte JN, Grimm M, Shinde NV, Nolan T, Worthen-Chaudhari L, Williams NO, Lustberg MB (2022) Updates in the treatment of chemotherapy-induced peripheral neuropathy. Curr Treat Options Oncol 23(1):29–42. https://doi.org/10.1007/s11864-021-00926-0
Hershman DL, Lacchetti C, Dworkin RH, Lavoie Smith EM, Bleeker J, Cavaletti G, Chauhan C, Gavin P et al (2014) Prevention and management of chemotherapy-induced peripheral neuropathy in survivors of adult cancers: American Society of Clinical Oncology clinical practice guideline. J Clin Oncol 32(18):1941–1967. https://doi.org/10.1200/JCO.2013.54.0914
He L, Hannon GJ (2004) MicroRNAs: small RNAs with a big role in gene regulation. Nat Rev Genet 5(7):522–531
Mendell JT, Olson EN (2012) MicroRNAs in stress signaling and human disease. Cell 148(6):1172–1187. https://doi.org/10.1016/j.cell.2012.02.005
Li B, Cao Y, Sun M, Feng H (2021) Expression, regulation, and function of exosome-derived miRNAs in cancer progression and therapy. FASEB J 35(10):e21916. https://doi.org/10.1096/fj.202100294RR
Lu TX, Rothenberg ME (2018) MicroRNA. J Allergy Clin Immunol 141(4):1202–1207. https://doi.org/10.1016/j.jaci.2017.08.034
Friedman RC, Farh KK, Burge CB, Bartel DP (2009) Most mammalian mRNAs are conserved targets of microRNAs. Genome Res 19(1):92–105. https://doi.org/10.1101/gr.082701.108
Kaur P, Kotru S, Singh S, Munshi A (2022) Role of miRNAs in diabetic neuropathy: mechanisms and possible interventions. Mol Neurobiol 59(3):1836–1849. https://doi.org/10.1007/s12035-021-02662-w
Wang Z, Liu F, Wei M, Qiu Y, Ma C, Shen L, Huang Y (2018) Chronic constriction injury-induced microRNA-146a-5p alleviates neuropathic pain through suppression of IRAK1/TRAF6 signaling pathway. J Neuroinflammation 15(1):179. https://doi.org/10.1186/s12974-018-1215-4
Pan Z, Shan Q, Gu P, Wang XM, Tai LW, Sun M, Luo X, Sun L et al (2018) miRNA-23a/CXCR4 regulates neuropathic pain via directly targeting TXNIP/NLRP3 inflammasome axis. J Neuroinflammation 15(1):29. https://doi.org/10.1186/s12974-018-1073-0
Eacker SM, Dawson TM, Dawson VL (2013) The interplay of microRNA and neuronal activity in health and disease. Front Cell Neurosci 7:136. https://doi.org/10.3389/fncel.2013.00136
Chen O, Donnelly CR, Ji R-R (2020) Regulation of pain by neuro-immune interactions between macrophages and nociceptor sensory neurons. Curr Opin Neurobiol 62:17–25. https://doi.org/10.1016/j.conb.2019.11.006
Khan NZ, Cao T, He J, Ritzel RM, Li Y, Henry RJ, Colson C, Stoica BA et al (2021) Spinal cord injury alters microRNA and CD81+ exosome levels in plasma extracellular nanoparticles with neuroinflammatory potential. Brain Behav Immun 92:165–183. https://doi.org/10.1016/j.bbi.2020.12.007
Jiang B-C, Cao D-L, Zhang X, Zhang Z-J, He L-N, Li C-H, Zhang W-W, Wu X-B et al (2016) CXCL13 drives spinal astrocyte activation and neuropathic pain via CXCR5. J Clin Invest 126(2):745–761. https://doi.org/10.1172/JCI81950
Tam Tam S, Bastian I, Zhou XF, Vander Hoek M, Michael MZ, Gibbins IL, Haberberger RV (2011) MicroRNA-143 expression in dorsal root ganglion neurons. Cell Tissue Res 346(2):163–173. https://doi.org/10.1007/s00441-011-1263-x
Polomano RC, Mannes AJ, Clark US, Bennett GJ (2001) A painful peripheral neuropathy in the rat produced by the chemotherapeutic drug, paclitaxel. Pain 94(3):293–304. https://doi.org/10.1016/S0304-3959(01)00363-3
Bolger AM, Lohse M, Usadel B (2014) Trimmomatic: a flexible trimmer for Illumina sequence data. Bioinformatics 30(15):2114–2120. https://doi.org/10.1093/bioinformatics/btu170
Kim D, Langmead B, Salzberg SL (2015) HISAT: a fast spliced aligner with low memory requirements. Nat Methods 12(4):357–360. https://doi.org/10.1038/nmeth.3317
Love MI, Huber W, Anders S (2014) Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol 15(12):550
Yu G, Wang L-G, Han Y, He Q-Y (2012) clusterProfiler: an R package for comparing biological themes among gene clusters. OMICS 16(5):284–287. https://doi.org/10.1089/omi.2011.0118
Ru Y, Kechris KJ, Tabakoff B, Hoffman P, Radcliffe RA, Bowler R, Mahaffey S, Rossi S et al (2014) The multiMiR R package and database: integration of microRNA-target interactions along with their disease and drug associations. Nucleic Acids Res 42(17):e133. https://doi.org/10.1093/nar/gku631
Li J-H, Liu S, Zhou H, Qu L-H, Yang J-H (2014) starBase v2.0: decoding miRNA-ceRNA, miRNA-ncRNA and protein-RNA interaction networks from large-scale CLIP-Seq data. Nucleic Acids Res 42 (Database issue):D92-D97. https://doi.org/10.1093/nar/gkt1248
Yu G, Li F, Qin Y, Bo X, Wu Y, Wang S (2010) GOSemSim: an R package for measuring semantic similarity among GO terms and gene products. Bioinformatics 26(7):976–978. https://doi.org/10.1093/bioinformatics/btq064
Finotello F, Trajanoski Z (2018) Quantifying tumor-infiltrating immune cells from transcriptomics data. Cancer Immunol Immunother 67(7):1031–1040. https://doi.org/10.1007/s00262-018-2150-z
Bindea G, Mlecnik B, Tosolini M, Kirilovsky A, Waldner M, Obenauf AC, Angell H, Fredriksen T et al (2013) Spatiotemporal dynamics of intratumoral immune cells reveal the immune landscape in human cancer. Immunity 39(4):782–795. https://doi.org/10.1016/j.immuni.2013.10.003
Russ DE, Cross RBP, Li L, Koch SC, Matson KJE, Yadav A, Alkaslasi MR, Lee DI et al (2021) A harmonized atlas of mouse spinal cord cell types and their spatial organization. Nat Commun 12(1):5722. https://doi.org/10.1038/s41467-021-25125-1
Sathyamurthy A, Johnson KR, Matson KJE, Dobrott CI, Li L, Ryba AR, Bergman TB, Kelly MC et al (2018) Massively parallel single nucleus transcriptional profiling defines spinal cord neurons and their activity during behavior. Cell Rep 22(8):2216–2225. https://doi.org/10.1016/j.celrep.2018.02.003
Hayashi M, Hinckley CA, Driscoll SP, Moore NJ, Levine AJ, Hilde KL, Sharma K, Pfaff SL (2018) Graded arrays of spinal and supraspinal V2a interneuron subtypes underlie forelimb and hindlimb motor control. Neuron 97(4). https://doi.org/10.1016/j.neuron.2018.01.023
Häring M, Zeisel A, Hochgerner H, Rinwa P, Jakobsson JET, Lönnerberg P, La Manno G, Sharma N et al (2018) Neuronal atlas of the dorsal horn defines its architecture and links sensory input to transcriptional cell types. Nat Neurosci 21(6):869–880. https://doi.org/10.1038/s41593-018-0141-1
Rosenberg AB, Roco CM, Muscat RA, Kuchina A, Sample P, Yao Z, Graybuck LT, Peeler DJ et al (2018) Single-cell profiling of the developing mouse brain and spinal cord with split-pool barcoding. Science 360(6385):176–182. https://doi.org/10.1126/science.aam8999
Zeisel A, Hochgerner H, Lönnerberg P, Johnsson A, Memic F, van der Zwan J, Häring M, Braun E, Borm LE, La Manno G, Codeluppi S, Furlan A, Lee K, Skene N, Harris KD, Hjerling-Leffler J, Arenas E, Ernfors P, Marklund U, Linnarsson S (2018) Molecular architecture of the mouse nervous system. Cell 174(4). https://doi.org/10.1016/j.cell.2018.06.021
Baek M, Menon V, Jessell TM, Hantman AW, Dasen JS (2019) Molecular logic of spinocerebellar tract neuron diversity and connectivity. Cell Rep 27(9):2620–2635. https://doi.org/10.1016/j.celrep.2019.04.113
Lu Y, Zhang J, Zeng F, Wang P, Guo X, Wang H, Qin Z, Tao T (2022) Human PMSCs-derived small extracellular vesicles alleviate neuropathic pain through miR-26a-5p/Wnt5a in SNI mice model. J Neuroinflammation 19(1):221. https://doi.org/10.1186/s12974-022-02578-9
Aczél T, Benczik B, Ágg B, Körtési T, Urbán P, Bauer W, Gyenesei A, Tuka B et al (2022) Disease- and headache-specific microRNA signatures and their predicted mRNA targets in peripheral blood mononuclear cells in migraineurs: role of inflammatory signalling and oxidative stress. J Headache Pain 23(1):113. https://doi.org/10.1186/s10194-022-01478-w
Yao C, Ren J, Huang R, Tang C, Cheng Y, Lv Z, Kong L, Fang S et al (2022) Transcriptome profiling of microRNAs reveals potential mechanisms of manual therapy alleviating neuropathic pain through microRNA-547-3p-mediated Map4k4/NF-κb signaling pathway. J Neuroinflammation 19(1):211. https://doi.org/10.1186/s12974-022-02568-x
Kuner R (2010) Central mechanisms of pathological pain. Nat Med 16(11):1258–1266. https://doi.org/10.1038/nm.2231
Li ZZ, Han WJ, Sun ZC, Chen Y, Sun JY, Cai GH, Liu WN, Wang TZ, Xie YD, Mao HH, Wang F, Ma SB, Wang FD, Xie RG, Wu SX, Luo C (2021) Extracellular matrix protein laminin β1 regulates pain sensitivity and anxiodepression-like behaviors in mice. J Clin Invest 131(15):e146323. https://doi.org/10.1172/JCI146323
Tajerian M, Hung V, Nguyen H, Lee G, Joubert L-M, Malkovskiy AV, Zou B, Xie S et al (2018) The hippocampal extracellular matrix regulates pain and memory after injury. Mol Psychiatry 23(12):2302–2313. https://doi.org/10.1038/s41380-018-0209-z
Cristino L, Bisogno T, Di Marzo V (2020) Cannabinoids and the expanded endocannabinoid system in neurological disorders. Nat Rev Neurol 16(1):9–29. https://doi.org/10.1038/s41582-019-0284-z
Buisseret B, Guillemot-Legris O, Ben Kouidar Y, Paquot A, Muccioli GG, Alhouayek M (2021) Effects of R-flurbiprofen and the oxygenated metabolites of endocannabinoids in inflammatory pain mice models. FASEB J 35(4):e21411. https://doi.org/10.1096/fj.202002468R
Smillie CL, Sirey T, Ponting CP (2018) Complexities of post-transcriptional regulation and the modeling of ceRNA crosstalk. Crit Rev Biochem Mol Biol 53(3):231–245. https://doi.org/10.1080/10409238.2018.1447542
Qi X, Zhang D-H, Wu N, Xiao J-H, Wang X, Ma W (2015) ceRNA in cancer: possible functions and clinical implications. J Med Genet 52(10):710–718. https://doi.org/10.1136/jmedgenet-2015-103334
Tu C, Wang L, Wei L, Jiang Z (2022) The role of circular RNA in diabetic nephropathy. Int J Med Sci 19(5):916–923. https://doi.org/10.7150/ijms.71648
Moreno-García L, López-Royo T, Calvo AC, Toivonen JM, de la Torre M, Moreno-Martínez L, Molina N, Aparicio P, Zaragoza P, Manzano R, Osta R (2020) Competing endogenous RNA networks as biomarkers in neurodegenerative diseases. Int J Mol Sci 21(24):9582. https://doi.org/10.3390/ijms21249582
Xian S, Ding R, Li M, Chen F (2021) LncRNA NEAT1/miR-128–3p/AQP4 axis regulating spinal cord injury-induced neuropathic pain progression. J Neuroimmunol 351:577457. https://doi.org/10.1016/j.jneuroim.2020.577457
Chen P, Wang C, Lin D, Li B, Ye S, Qu J, Wang W (2021) Identification of Slc6a19os and SOX11 as two novel essential genes in neuropathic pain using integrated bioinformatic analysis and experimental verification. Front Neurosci 15:627945. https://doi.org/10.3389/fnins.2021.627945
Shao J, Wang J, Huang J, Liu C, Pan Y, Guo Q, Zou W (2018) Identification of lncRNA expression profiles and ceRNA analysis in the spinal cord of morphine-tolerant rats. Mol Brain 11(1):21. https://doi.org/10.1186/s13041-018-0365-8
Li H, Fan L, Zhang Y, Cao Y, Liu X (2020) SNHG16 aggravates chronic constriction injury-induced neuropathic pain in rats via binding with miR-124-3p and miR-141-3p to upregulate JAG1. Brain Res Bull 165:228–237. https://doi.org/10.1016/j.brainresbull.2020.09.025
Fumagalli G, Monza L, Cavaletti G, Rigolio R, Meregalli C (2020) Neuroinflammatory process involved in different preclinical models of chemotherapy-induced peripheral neuropathy. Front Immunol 11:626687. https://doi.org/10.3389/fimmu.2020.626687
Lees JG, Makker PGS, Tonkin RS, Abdulla M, Park SB, Goldstein D, Moalem-Taylor G (2017) Immune-mediated processes implicated in chemotherapy-induced peripheral neuropathy. Eur J Cancer 73:22–29. https://doi.org/10.1016/j.ejca.2016.12.006
Skaper SD, Giusti P, Facci L (2012) Microglia and mast cells: two tracks on the road to neuroinflammation. FASEB J 26(8):3103–3117. https://doi.org/10.1096/fj.11-197194
Luo X, Huh Y, Bang S, He Q, Zhang L, Matsuda M, Ji R-R (2019) Macrophage toll-like receptor 9 contributes to chemotherapy-induced neuropathic pain in male mice. J Neurosci 39(35):6848–6864. https://doi.org/10.1523/JNEUROSCI.3257-18.2019
Zhang H, Li Y, de Carvalho-Barbosa M, Kavelaars A, Heijnen CJ, Albrecht PJ, Dougherty PM (2016) Dorsal root ganglion infiltration by macrophages contributes to paclitaxel chemotherapy-induced peripheral neuropathy. J Pain 17(7):775–86. https://doi.org/10.1016/j.jpain.2016.02.011
Krukowski K, Eijkelkamp N, Laumet G, Hack CE, Li Y, Dougherty PM, Heijnen CJ, Kavelaars A (2016) CD8+ T cells and endogenous IL-10 are required for resolution of chemotherapy-induced neuropathic pain. J Neurosci 36(43):11074–11083
Singh SK, Krukowski K, Laumet GO, Weis D, Alexander JF, Heijnen CJ, Kavelaars A (2022) CD8+ T cell-derived IL-13 increases macrophage IL-10 to resolve neuropathic pain. JCI Insight 7(5):e154194. https://doi.org/10.1172/jci.insight.154194
Yang J, Yaron A, Liu K (2022) Editorial: neuroimmune interactions in peripheral neuropathy. Front Mol Neurosci 15:929081. https://doi.org/10.3389/fnmol.2022.929081
Strober W, Fuss IJ (2011) Proinflammatory cytokines in the pathogenesis of inflammatory bowel diseases. Gastroenterology 140(6):1756–1767. https://doi.org/10.1053/j.gastro.2011.02.016
Bettelli E, Carrier Y, Gao W, Korn T, Strom TB, Oukka M, Weiner HL, Kuchroo VK (2006) Reciprocal developmental pathways for the generation of pathogenic effector TH17 and regulatory T cells. Nature 441(7090):235–238
Kämpe A, Knebel A, Carlson R, Rohn K, Tipold A (2021) Evaluation of the involvement of Th17-cells in the pathogenesis of canine spinal cord injury. PLoS One 16(9):e0257442. https://doi.org/10.1371/journal.pone.0257442
Huo W, Liu Y, Lei Y, Zhang Y, Huang Y, Mao Y, Wang C, Ye S et al (2019) Imbalanced spinal infiltration of Th17/Treg cells contributes to bone cancer pain via promoting microglial activation. Brain Behav Immun 79:139–151. https://doi.org/10.1016/j.bbi.2019.01.024
Quick ML, Wong L, Mukherjee S, Done JD, Schaeffer AJ, Thumbikat P (2013) Th1-Th17 cells contribute to the development of uropathogenic Escherichia coli-induced chronic pelvic pain. PLoS One 8(4):e60987. https://doi.org/10.1371/journal.pone.0060987
Luo H, Liu HZ, Zhang WW, Matsuda M, Lv N, Chen G, Xu ZZ, Zhang YQ (2019) Interleukin-17 regulates neuron-glial communications, synaptic transmission, and neuropathic pain after chemotherapy. Cell Rep 29(8):2384–2397. https://doi.org/10.1016/j.celrep.2019.10.085
Gabrilovich DI, Nagaraj S (2009) Myeloid-derived suppressor cells as regulators of the immune system. Nat Rev Immunol 9(3):162–174. https://doi.org/10.1038/nri2506
Zhang T, He Y, Man GCW, Ding Y, Wang CC, Chung JPW (2023) Myeloid-derived suppressor cells: a new emerging player in endometriosis. Int Rev Cell Mol Biol 375:191–220. https://doi.org/10.1016/bs.ircmb.2022.11.004
Oshima Y, Sano M, Kajiwara I, Ichimaru Y, Itaya T, Kuramochi T, Hayashi E, Kim J et al (2022) Midazolam exhibits antitumour and anti-inflammatory effects in a mouse model of pancreatic ductal adenocarcinoma. Br J Anaesth 128(4):679–690. https://doi.org/10.1016/j.bja.2021.12.042
Salzer JL (2015) Schwann cell myelination. Cold Spring Harb Perspect Biol 7(8):a020529. https://doi.org/10.1101/cshperspect.a020529
Shy ME (2006) Peripheral neuropathies caused by mutations in the myelin protein zero. J Neurol Sci 242(1–2):55–66
Otani Y, Ohno N, Cui J, Yamaguchi Y, Baba H (2020) Upregulation of large myelin protein zero leads to Charcot-Marie-Tooth disease-like neuropathy in mice. Commun Biol 3(1):121. https://doi.org/10.1038/s42003-020-0854-z
Keller MP, Chance PF (1999) Inherited neuropathies: from gene to disease. Brain Pathol 9(2):327–341
Agrahari A, George Priya Doss C (2015) Impact of I30T and I30M substitution in MPZ gene associated with Dejerine-Sottas syndrome type B (DSSB): a molecular modeling and dynamics. J Theor Biol 382:23–33. https://doi.org/10.1016/j.jtbi.2015.06.019
Ma B, Liu X, Huang X, Ji Y, Jin T, Ma K (2018) Translocator protein agonist Ro5-4864 alleviates neuropathic pain and promotes remyelination in the sciatic nerve. Mol Pain 14:1744806917748019. https://doi.org/10.1177/1744806917748019
Hao G-M, Liu Y-G, Wu Y, Xing W, Guo S-Z, Wang Y, Wang Z-L, Li C et al (2017) The protective effect of the active components of ERPC on diabetic peripheral neuropathy in rats. J Ethnopharmacol 202:162–171. https://doi.org/10.1016/j.jep.2017.03.015
Song W, Jiang W, Wang C, Xie J, Liang X, Sun Y, Gong L, Liu W et al (2019) Jinmaitong, a traditional Chinese compound prescription, ameliorates the streptozocin-induced diabetic peripheral neuropathy rats by increasing sciatic nervE IGF-1 and IGF-1R expression. Front Pharmacol 10:255. https://doi.org/10.3389/fphar.2019.00255
Funding
This study was supported by the National Natural Science Foundation of China (81870878, 8217102207, 82101348), and Guangdong Basic and Applied Basic Research Foundation (2019B151502010, 2022B1515120026, 2020A1515110087).
Author information
Authors and Affiliations
Contributions
XJD and HJX conceived the study. YXH, HXQ, and LWC performed bioinformatics and statistical data analyses. YXH and YF constructed the CINP models and collected the tissues. YXH, LWC, YYQ, WLJ, and TXL performed the experiments. ZWA helped perform the analysis with constructive discussions. YXH, HXQ, and LWC wrote the manuscript. All the authors approved the manuscript.
Corresponding authors
Ethics declarations
Ethics Approval and Consent to Participate
All experimental animal procedures were approved by Sun Yat-sen University Cancer Center Animal Care and Use Committee and conformed to the National Institutes of Health guidelines on the care and ethical treatment of animals.
Consent for Publication
All the authors read and approved the publication of this manuscript.
Competing Interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Yang, X., Huang, X., Lu, W. et al. Transcriptome Profiling of miRNA-mRNA Interactions and Associated Mechanisms in Chemotherapy-Induced Neuropathic Pain. Mol Neurobiol 60, 5672–5690 (2023). https://doi.org/10.1007/s12035-023-03398-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12035-023-03398-5